B1378
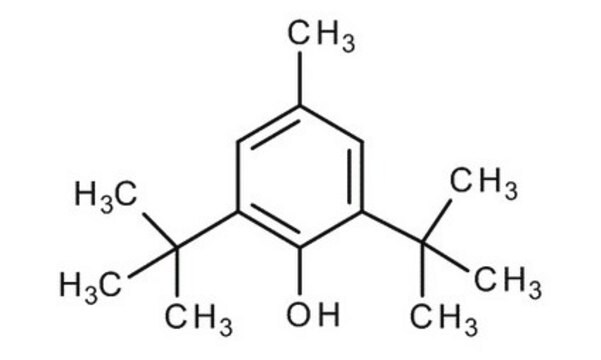
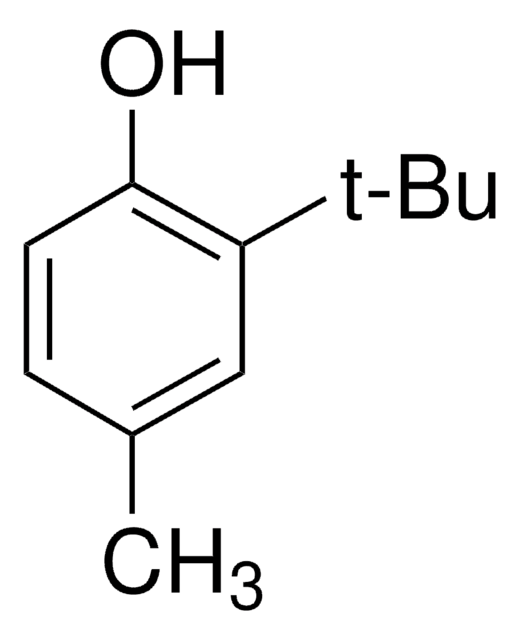
2,6-Di-tert-butyl-4-methylphenol
≥99.0% (GC), powder
Synonym(s):
2,6-Di-tert-butyl-p-cresol, BHT, Butylated hydroxytoluene, Butylhydroxytoluene, DBPC
About This Item
Recommended Products
vapor density
7.6 (vs air)
Quality Level
vapor pressure
<0.01 mmHg ( 20 °C)
Assay
≥99.0% (GC)
form
powder
autoignition temp.
878 °F
bp
265 °C (lit.)
mp
69-73 °C (lit.)
solubility
ethanol: 100 mg/mL
vegetable oils: soluble
SMILES string
Cc1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C15H24O/c1-10-8-11(14(2,3)4)13(16)12(9-10)15(5,6)7/h8-9,16H,1-7H3
InChI key
NLZUEZXRPGMBCV-UHFFFAOYSA-N
Gene Information
human ... CAPN1(823)
rat ... Capn1(29153) , Nos1(24598)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Thermal decomposition characteristics of BHT and its peroxide (BHTOOH): BHT is highlighted for its excellent antioxidant properties, which degrade into BHTOOH under specific conditions (S Dai, et al., 2024).
- Relaxation mechanisms in low-stress polymer networks: BHT is utilized in polymer networks to assess its effects on the mechanical properties and durability of dental composites (SH Lewis, et al., 2024).
- Compatibility and safety of labels for pharmaceutical packaging: The study found BHT in labels, indicating its widespread use in pharmaceutical packaging due to its stabilizing properties (C Fu, et al., 2023).
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
260.6 °F - open cup
Flash Point(C)
127 °C - open cup
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
While quantitative analysis was performed for Vitamins D2 and D3, the samples were scanned for the presence of the 25-hydroxy metabolites.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service