92893
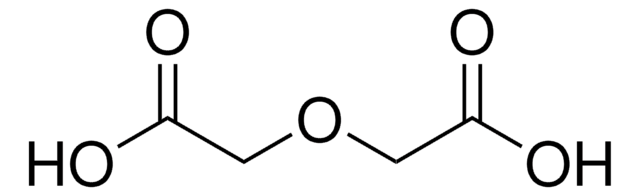
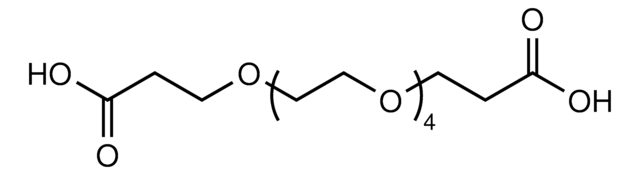
3,6,9-Trioxaundecanedioic acid
technical, ≥70% (T)
Synonym(s):
O,O′-Oxydiethylene-diglycolic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H14O7
CAS Number:
Molecular Weight:
222.19
Beilstein:
1962084
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥70% (T)
form
liquid
reaction suitability
reaction type: Pegylations
reagent type: cross-linking reagent
impurities
~10% water
refractive index
n20/D 1.470 (lit.)
density
1.3 g/mL at 20 °C (lit.)
SMILES string
OC(=O)COCCOCCOCC(O)=O
InChI
1S/C8H14O7/c9-7(10)5-14-3-1-13-2-4-15-6-8(11)12/h1-6H2,(H,9,10)(H,11,12)
InChI key
HJZZQNLKBWJYPD-UHFFFAOYSA-N
General description
3,6,9-Trioxaundecanedioic, acid also known as tetraglycolic acid, is a water miscible hydrophilic cross-linking reagent, widely utilized in PEGylation reactions.
Application
3,6,9-Trioxaundecanedioic acid is used as a cross linking agent in the synthesis of biodegradable, kojic acid-based poly(carbonate-esters).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jonathan J Faig et al.
Biomacromolecules, 18(2), 363-373 (2016-12-28)
Kojic acid (KA) is a naturally occurring fungal metabolite that is utilized as a skin-lightener and antibrowning agent owing to its potent tyrosinase inhibition activity. While efficacious, KA's inclination to undergo pH-mediated, thermal-, and photodegradation reduces its efficacy, necessitating stabilizing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)