900402
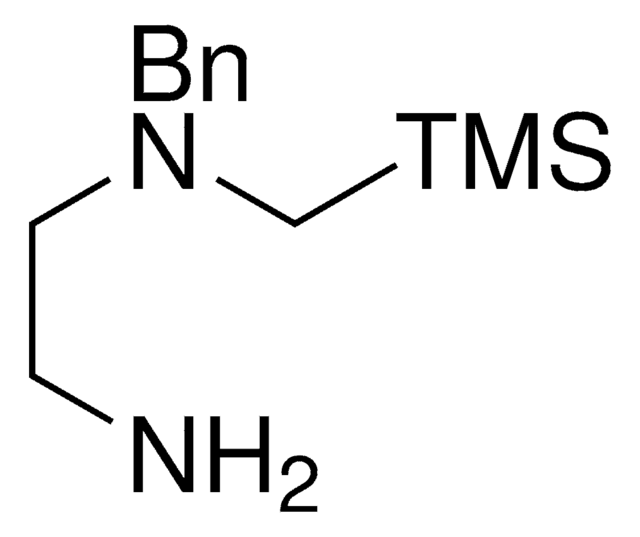
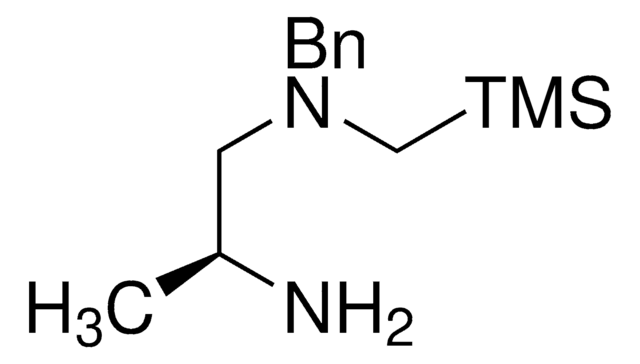
SLAP HydroQuinoxaline
95%
Synonym(s):
trans-N1-Benzyl-N1-((trimethylsilyl)methyl)cyclohexane-1,2-diamine
About This Item
Recommended Products
Assay
95%
form
liquid
refractive index
n/D 1.5222
density
0.961 g/mL
functional group
amine
phenyl
storage temp.
−20°C
Application
Other Notes
- Technology Spotlight: SLAP Reagents for Piperazine Synthesis
- Silicon Amine Reagents for the Photocatalytic Synthesis of Piperazines from Aldehydes and Ketones
- Lewis Acid Induced Toggle from Ir(II) to Ir(IV) Pathways in Photocatalytic Reactions: Synthesis of Thiomorpholines and Thiazepanes from Aldehydes and SLAP Reagents.
- Continuous Flow Synthesis of Morpholines and Oxazepanes with Silicon Amine Protocol (SLAP) Reagents and Lewis Acid Facilitated Photoredox Catalysis
related product
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
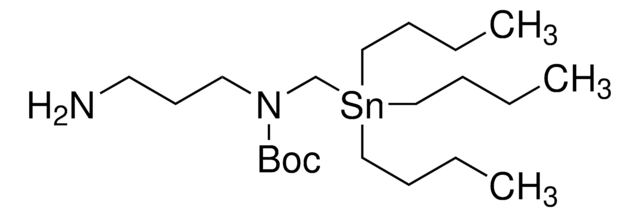
The Bode group has developed SnAP (stannyl amine protocol) reagents that cross-couple with aldehydes and ketones to provide one-step access to a wide variety of saturated N-heterocycles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/309/053/0823f035-245c-433d-b033-2eca2d931c67/640/0823f035-245c-433d-b033-2eca2d931c67.png)