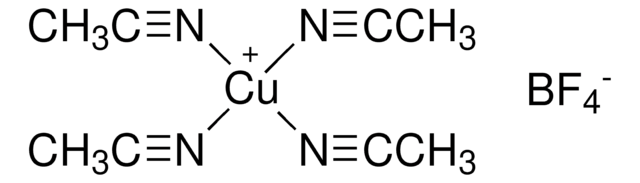
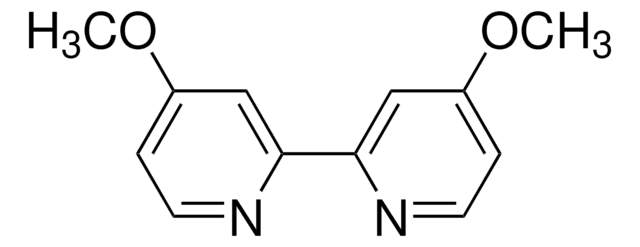
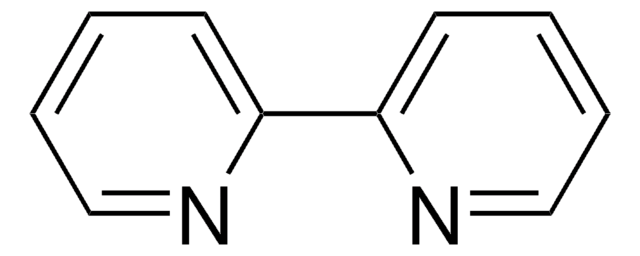
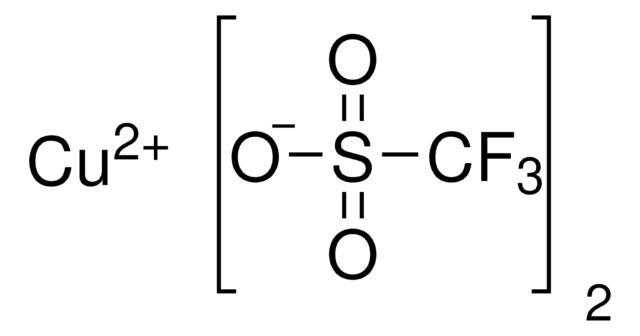796549
Stahl Aerobic Oxidation TEMPO solution
0.2 M in acetonitrile, Solution for Oxidation of Primary Alcohols
About This Item
Recommended Products
Quality Level
form
liquid
reaction suitability
reagent type: oxidant
concentration
0.2 M in acetonitrile
storage temp.
2-8°C
SMILES string
CN1C=CN=C1.CC2(C)CCCC(C)(C)N2[O].C3(C4=NC=CC=C4)=NC=CC=C3
InChI
1S/C10H8N2.C9H18NO.C4H6N2/c1-3-7-11-9(5-1)10-6-2-4-8-12-10;1-8(2)6-5-7-9(3,4)10(8)11;1-6-3-2-5-4-6/h1-8H;5-7H2,1-4H3;2-4H,1H3
InChI key
BQFURWVGIDXRNB-UHFFFAOYSA-N
General description
Application
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1C
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
35.6 °F
Flash Point(C)
2.0 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Alcohol oxidation is one of the most frequently performed oxidation reactions in organic chemistry. The aldehyde and ketone products of alcohol oxidation are useful intermediates en route to complex molecules.
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service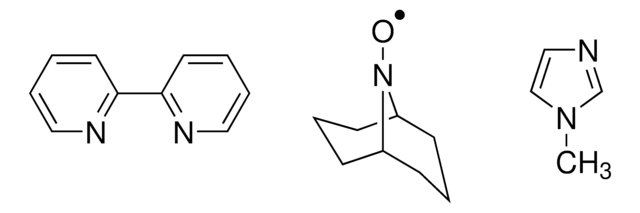
![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)