692484
(R)-DTBM-SEGPHOS®
Synonym(s):
(R)-(−)-5,5′-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole, [(4R)-(4,4′-bi-1,3-benzodioxole)-5,5′-diyl]bis[bis(3,5-di-tert-butyl-4-methoxyphenyl)phosphine]
About This Item
Recommended Products
form
powder
Quality Level
InChI
1S/C74H100O8P2/c1-67(2,3)47-33-43(34-48(61(47)75-25)68(4,5)6)83(44-35-49(69(7,8)9)62(76-26)50(36-44)70(10,11)12)57-31-29-55-65(81-41-79-55)59(57)60-58(32-30-56-66(60)82-42-80-56)84(45-37-51(71(13,14)15)63(77-27)52(38-45)72(16,17)18)46-39-53(73(19,20)21)64(78-28)54(40-46)74(22,23)24/h29-40H,41-42H2,1-28H3
InChI key
ZNORAFJUESSLTM-UHFFFAOYSA-N
Application
- [3,3]-Sigmatropic rearrangements using cyclopropane probes
- Asymmetric intramolecular hydroacylation of ketoaldehydes
Reactant involved in:
- The synthesis of gold-diphosphine complexes for use as catalysts
- Cycloaddition of allenenes to yield alkylidenecyclobutanes
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service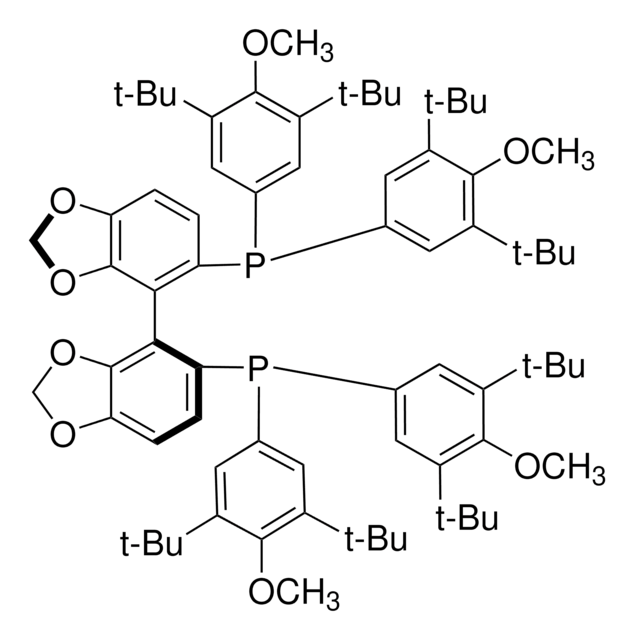
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)

![(R)-(6,6′-Dimethoxybiphenyl-2,2′-diyl)bis[bis(3,5-di-tert-butylphenyl)phosphine] ≥97%, optical purity ee: ≥99%](/deepweb/assets/sigmaaldrich/product/structures/389/033/e3c61de2-e5d4-4141-be57-0eb722850a37/640/e3c61de2-e5d4-4141-be57-0eb722850a37.png)
![(+)-1,2-Bis[(2S,5S)-2,5-dimethylphospholano]benzene kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)