679704
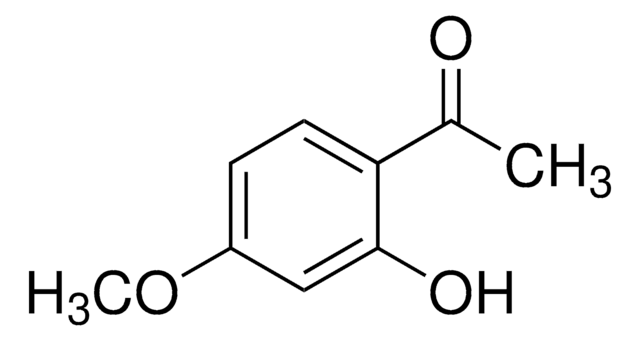
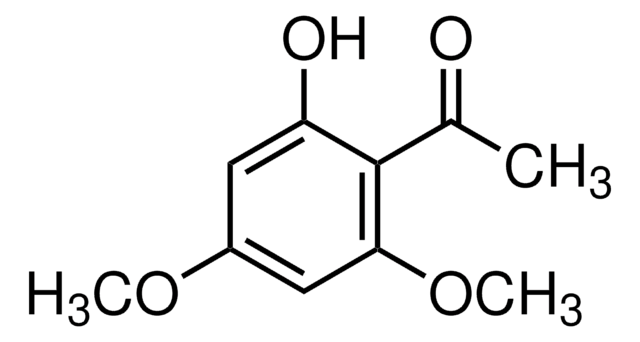
3′-Hydroxy-4′-methoxyacetophenone
97%
Synonym(s):
1-(3-hydroxy-4-methoxyphenyl)ethanone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H10O3
CAS Number:
Molecular Weight:
166.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
88-92 °C
functional group
ketone
SMILES string
COc1ccc(cc1O)C(C)=O
InChI
1S/C9H10O3/c1-6(10)7-3-4-9(12-2)8(11)5-7/h3-5,11H,1-2H3
InChI key
YLTGFGDODHXMFB-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lee Fielding et al.
Magnetic resonance in chemistry : MRC, 49(7), 405-412 (2011-05-07)
The association constants for the interactions of 2-hydroxy-4-methoxyacetophenone, 2-hydroxy-5-methoxyacetophenone, 2-hydroxy-6-methoxyacetophenone, 3-hydroxy-4-methoxyacetophenone and 4-hydroxy-3-methoxyacetophenone with β-cyclodextrin in water were measured by (1)H NMR and by isothermal titration calorimetry. Very good agreement was obtained between the different methods. The errors associated with
F Z Sun et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 18(6), 362-363 (1993-06-01)
3-Hydroxy-4-methoxy-acetophenone (HMA) isolated from Cynanchum paniculatum was found to have analgesic effect and inhibitory action on the gastro-intestinal motility. The effect and action were equivalent to that of paeonol, HMA displayed a very low degree of antibacterial activity against Escherichia
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service