54920
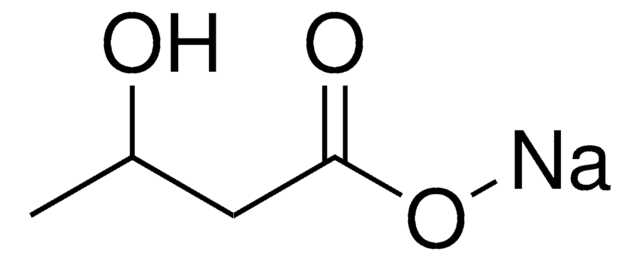
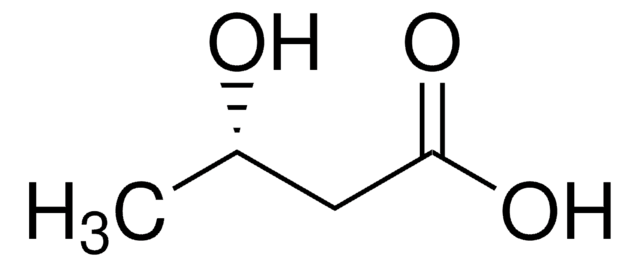
(R)-3-Hydroxybutyric acid
≥98.0% (T)
Synonym(s):
(3R)-3-Hydroxybutanoic acid, (3R)-Hydroxybutyrate, 3R-Hydroxybutanoic acid, D-β-Hydroxybutyrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
Beilstein:
1720568
EC Number:
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
optical activity
[α]20/D −25±1°, c = 6% in H2O
mp
49-50 °C (lit.)
functional group
carboxylic acid
hydroxyl
storage temp.
2-8°C
SMILES string
C[C@@H](O)CC(O)=O
InChI
1S/C4H8O3/c1-3(5)2-4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
WHBMMWSBFZVSSR-GSVOUGTGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(R)-3-Hydroxybutyric acid may be used in the preparation of copolymers by reacting with various hydroxyalkanoic acids.1 It may also be used in the preparation of (3R,4R)-4-acetoxy-3-[(R)-1-(formyloxy)ethyl]-2-azetidinone.2
Other Notes
Important chiral starting material; enantioselective reactions at the 2-, 3- and 4-positions via the cyclic acetal with aldehydes; preparation of (R)-β-butyrolactone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Journal of the American Chemical Society, 110, 4763-4763 (1988)
D. Seebach et al.
Modern Synthetic Methods, 4, 125-125 (1986)
Sodium-glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter-organ crosstalk.
Jin Hee Kim et al.
Diabetes, obesity & metabolism (2018-11-09)
To investigate sodium-glucose cotransporter 2 inhibitor (SGLT2i)-induced changes in ketogenic enzymes and transporters in normal and diabetic mice models. Normal mice were randomly assigned to receive either vehicle or SGLT2i (25 mg/kg/d by oral gavage) for 7 days. Diabetic mice were treated
A. Griesbech et al.
Helvetica Chimica Acta, 70, 1320-1320 (1987)
J L Hansen et al.
Clinical chemistry, 24(3), 475-479 (1978-03-01)
Methods are described for direct assays of lactate, pyruvate, beta-hydroxybutyrate, and acetoacetate in plasma with the GEMSAEC centrifugal analyzer. The methods for lactate, beta-hydroxybutyrate, and acetoacetate are kinetic and ratiometric, eliminating the need for specimen-blank assays. The pyruvate method is
Chromatograms
suitable for GCGlobal Trade Item Number
| SKU | GTIN |
|---|---|
| T212083-1EA | |
| 54920-1G-F | 4061832575100 |
| 54920-5G-F | 4061832896212 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)