All Photos(1)
About This Item
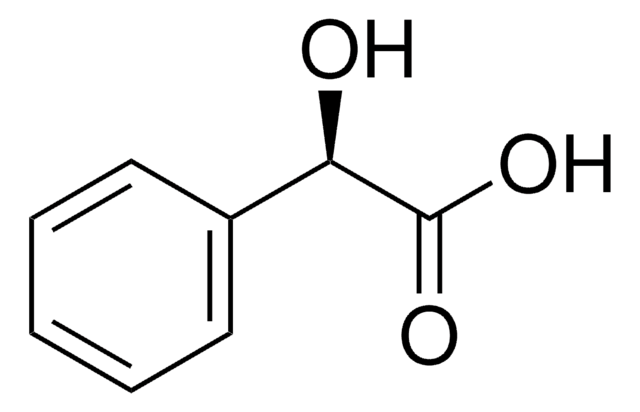
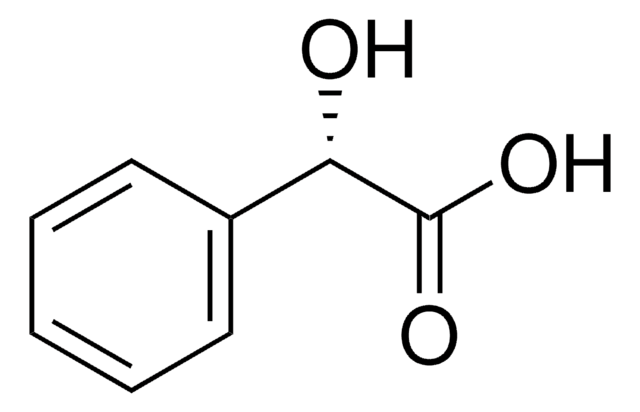
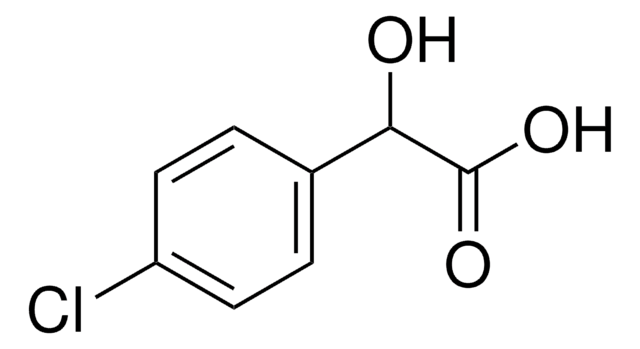
Linear Formula:
ClC6H4CH(OH)CO2H
CAS Number:
Molecular Weight:
186.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
90-93 °C (lit.)
functional group
carboxylic acid
chloro
hydroxyl
SMILES string
OC(C(O)=O)c1ccccc1Cl
InChI
1S/C8H7ClO3/c9-6-4-2-1-3-5(6)7(10)8(11)12/h1-4,7,10H,(H,11,12)
InChI key
RWOLDZZTBNYTMS-UHFFFAOYSA-N
General description
2-Chloromandelic acid (2-ClMA) is a mandelic acid derivative. A report based on its solid state-NMR, X-ray powder diffraction (XPRD) and Fourier transform infrared spectroscopy (FTIR) data reveals that in solid state 2-ClMA exists as a racemic compound. The study also suggests that the crystals of racemic 2-ClMA belongs the monoclinic space group P21/c. The efficiency of (R)-(+)-N-benzyl-1-phenylethylamine in resolving racemate 2-ClMA has been investigated.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Quan He et al.
Journal of pharmaceutical sciences, 98(5), 1835-1844 (2008-10-07)
The racemate and enantiomers of 2-chloromandelic acid were characterized by SS-NMR, XRPD, and FTIR. The binary melting point phase diagram was constructed by DSC (differential scanning calorimetry). The solid-state nature of 2-chloromandelic acid was identified to be a racemic compound.
Xin Yuan et al.
Biotechnology progress, 35(4), e2815-e2815 (2019-04-10)
Optically pure 2-chloromandelic acid (ClMA) is a very important chiral drug intermediate for synthesis of (S)-clopidogrel, belonging to the platelet aggregation inhibitor. Enantioselective resolution of (R,S)-2-chloromandelic acid was carried out in organic solvent through irreversible transesterification catalyzed by lipase AK
Yangfeng Peng et al.
Chirality, 24(5), 349-355 (2012-04-18)
During the resolution of 2-chloromandelic acid with (R)-(+)-N-benzyl-1-phenylethylamine, the crystals of the less soluble salt were grown, and their structure were determined and presented. The chiral discrimination mechanism was investigated by examining the weak intermolecular interactions (such as hydrogen bond
Shengqiang Tong et al.
Journal of separation science, 38(12), 2085-2092 (2015-04-14)
The chromatographic retention mechanism describing relationship between retention factor and concentration of Cu(2+) (l-phenylalanine)2 using chiral ligand mobile phase was investigated and eight mandelic acid derivatives were enantioseparated by chiral ligand exchange chromatography. The relationship between retention factor and concentration
Rafael Gozalbes et al.
Bioorganic & medicinal chemistry, 18(19), 7078-7084 (2010-09-03)
Solubility plays a very important role in the selection of compounds for drug screening. In this context, a QSAR model was developed for predicting water solubility of drug-like compounds. First, a set of relevant parameters for establishing a drug-like chemical
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service