All Photos(1)
About This Item
Empirical Formula (Hill Notation):
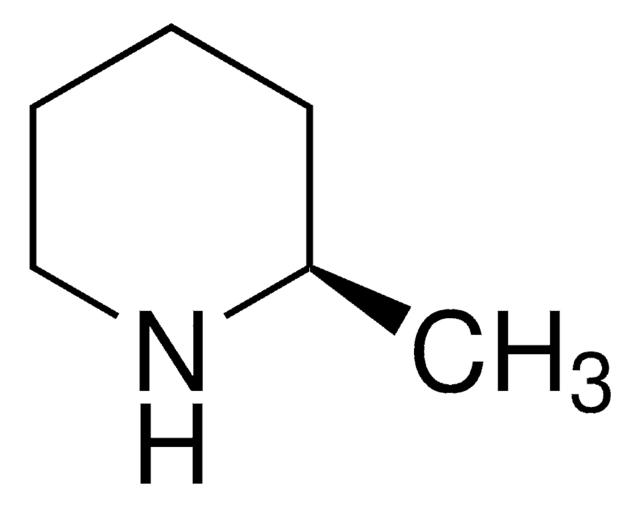
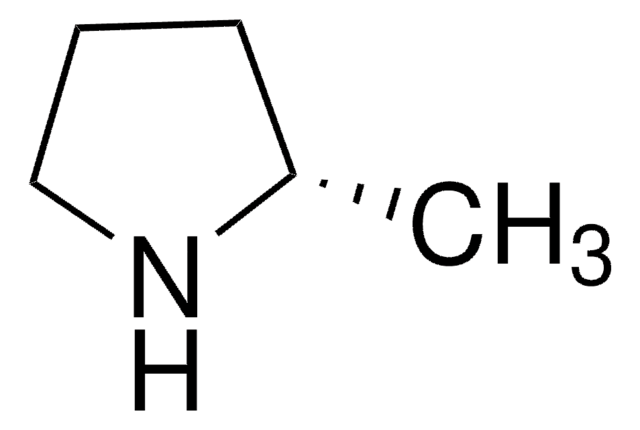
C6H13N
CAS Number:
Molecular Weight:
99.17
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D +35°, c = 3.5 in hexane
refractive index
n20/D 1.446 (lit.)
bp
117-121 °C (lit.)
density
0.823 g/mL at 25 °C (lit.)
SMILES string
C[C@H]1CCCCN1
InChI
1S/C6H13N/c1-6-4-2-3-5-7-6/h6-7H,2-5H2,1H3/t6-/m0/s1
InChI key
NNWUEBIEOFQMSS-LURJTMIESA-N
Related Categories
Application
(S)-(+)-2-Methylpiperidine has been used in the preparation of (S)-N-(3-(2-pipecolin-1-yl)propyl)phthalimide by reacting with N-(3-bromopropyl)phthalimide. It may also be used as a starting material in the multi-step synthesis of (2S,6S)-(+)-solenopsin A.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.0 °F - closed cup
Flash Point(C)
15.56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Noncyclam Tetraamines Inhibit CXC Chemokine Receptor Type 4 and Target Glioma-Initiating Cells.
Ros-Blanco L, et al.
Journal of Medicinal Chemistry, 55(17), 7560-7570 (2012)
Anodic Cyanation of (-)-N-Phenyl-2-Methylpiperidine: A Short Synthesis of (+)-Solenopsin A and (+)-Isosolenopsin A.
Girard N, et al.
Synthetic Communications, 35(5), 711-723 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service