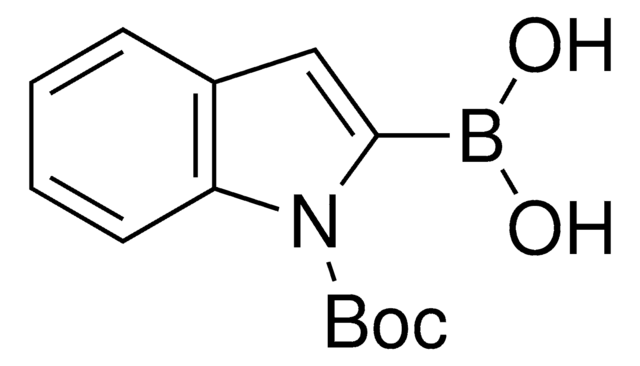
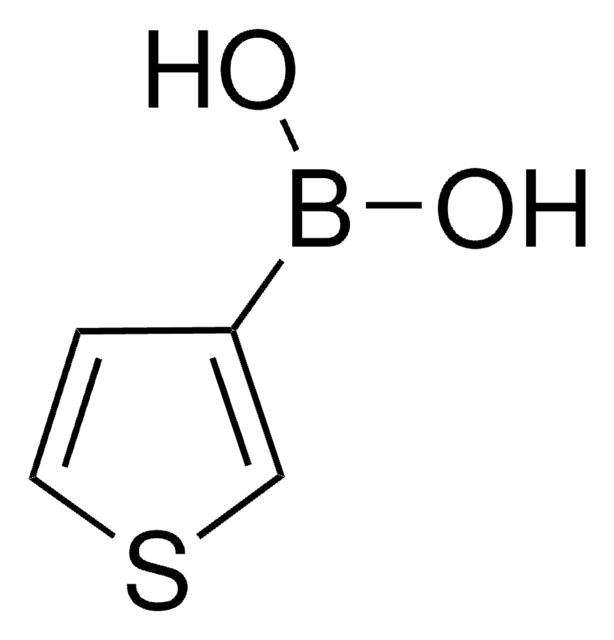
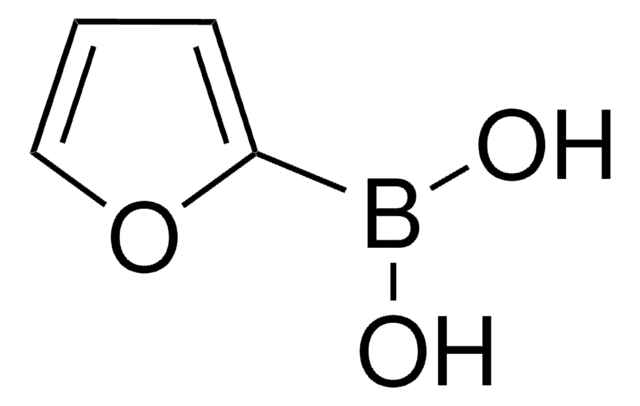
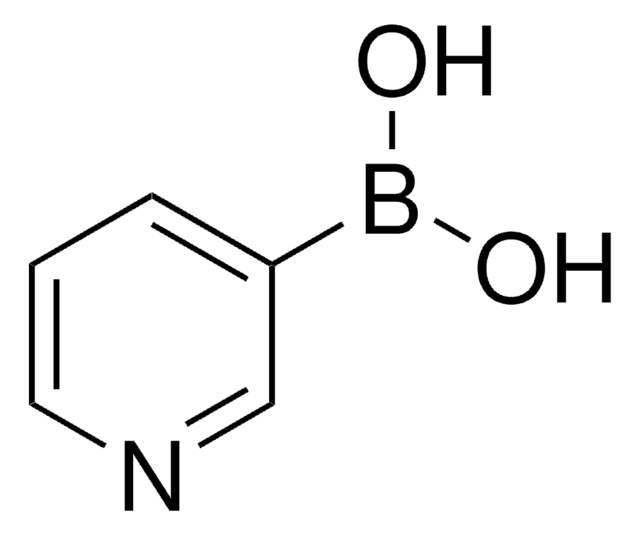
512117
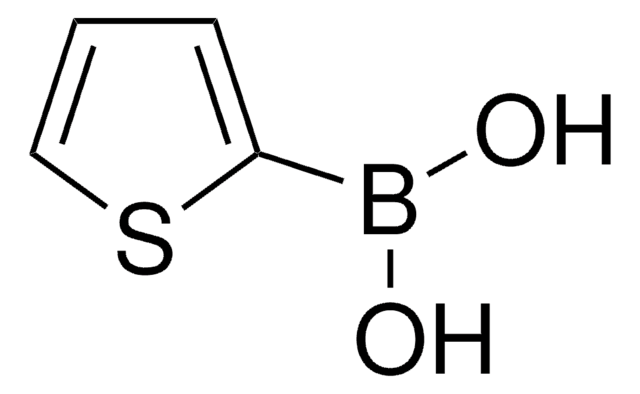
Benzo[b]thien-3-ylboronic acid
≥95.0%
Synonym(s):
Thianaphthene-3-boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H7BO2S
CAS Number:
Molecular Weight:
178.02
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
225-230 °C (lit.)
SMILES string
OB(O)c1csc2ccccc12
InChI
1S/C8H7BO2S/c10-9(11)7-5-12-8-4-2-1-3-6(7)8/h1-5,10-11H
InChI key
QVANIYYVZZLQJP-UHFFFAOYSA-N
Related Categories
Application
Benzo[b]thien-3-ylboronic acid can be used:
- To prepare thienyl substituted pyrimidine derivatives as potent antimycobacterial compounds.
- To prepare 3-O-protected 17-heteroaryl-3-hydroxyestra-1,3,5,16-tetraene-16-carbaldehyde, which in turn is used for the synthesis of heteroarenes-annelated estranes.
- As a substrate in the study of metal-free coupling reactions of allylic alcohols with heteroaryl boronic acids.
- As a starting material for the preparation of thienyl based quinoline and pyridine ligands, which are further used to synthesize platinum complexes.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, characterization, and photophysical properties of bismetalated platinum complexes with benzothiophene ligands
Anderson CM, et al.
Journal of Organometallic Chemistry, 882, 10-17 (2019)
Heteroareno-annelated estranes by triene cyclization
Watanabe M, et al.
open chemistry, 4(3), 375-402 (2006)
Articles
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)