462101
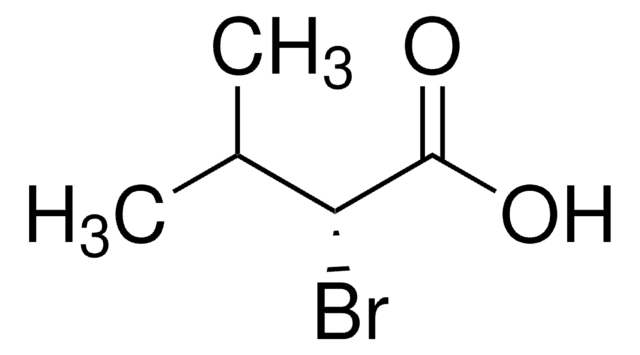
(S)-(−)-2-Bromo-3-methylbutyric acid
96%
Synonym(s):
(S)-2-Bromoisovaleric acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHCH(Br)CO2H
CAS Number:
Molecular Weight:
181.03
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
Recommended Products
Assay
96%
form
solid
optical activity
[α]22/D −21°, c = 37 in benzene
bp
90-100 °C/0.5 mmHg (lit.)
mp
39-44 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
CC(C)[C@H](Br)C(O)=O
InChI
1S/C5H9BrO2/c1-3(2)4(6)5(7)8/h3-4H,1-2H3,(H,7,8)/t4-/m0/s1
InChI key
UEBARDWJXBGYEJ-BYPYZUCNSA-N
Application
Ferroelectric liquid crystal derivatives, proline-valine pseudo dipeptides which are potent inhibitors of α-chymotrypsin, and β-turn peptidomimetics have been prepared using this bromoacid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sierra, T. et al.
Journal of the American Chemical Society, 114, 7645-7645 (1992)
Reed, P.E. Katzenellenbogen, J.A.
The Journal of Organic Chemistry, 56, 2624-2624 (1991)
Virgilio, A.A. et al.
Tetrahedron Letters, 37, 6961-6961 (1996)
M Polhuijs et al.
Biochemical pharmacology, 44(7), 1249-1253 (1992-10-06)
Glutathione (GSH) conjugation of the separate enantiomers of five 2-bromocarboxylic acids and some of their urea derivatives by rat liver GSH transferases (GSTs) was studied. The liver cytosolic fraction conjugated all compounds, except for (R)-2-bromoisovaleric acid, with a variable degree
M Polhuijs et al.
Biochemical pharmacology, 38(22), 3957-3962 (1989-11-15)
The glutathione (GSH) conjugation of (R)-and (S)-alpha-bromoisovaleric acid (BI) in the rat in vivo, and its stereoselectivity, have been characterized. After administration of racemic [1-14C]BI two radioactive metabolites were found in bile: only one of the possible diastereomeric BI-GSH conjugates
Chromatograms
application for HPLCGlobal Trade Item Number
| SKU | GTIN |
|---|---|
| 462101-25G | |
| 462101-5G | 4061832896113 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![4-tert-Butylcalix[4]arene 95%](/deepweb/assets/sigmaaldrich/product/structures/141/993/6110bb7e-274f-45f8-887a-f1841a7dae7c/640/6110bb7e-274f-45f8-887a-f1841a7dae7c.png)
![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)
![7-Bromo[1,2,4]triazolo[1,5-a]pyridine](/deepweb/assets/sigmaaldrich/product/structures/106/727/8445136c-b972-4b82-85e7-54c21e178da3/640/8445136c-b972-4b82-85e7-54c21e178da3.png)
![Calix[6]arene 97%](/deepweb/assets/sigmaaldrich/product/structures/310/111/14d1ce22-aecd-4c86-8304-5c017a1c1534/640/14d1ce22-aecd-4c86-8304-5c017a1c1534.png)