347833
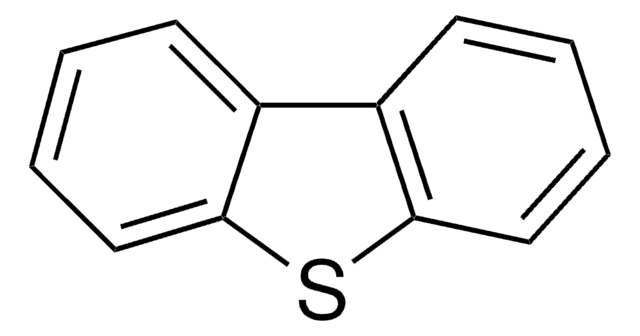
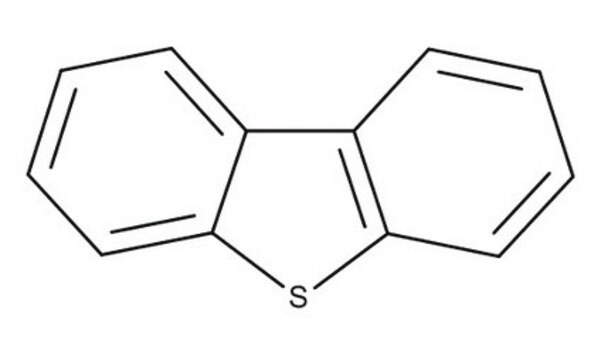
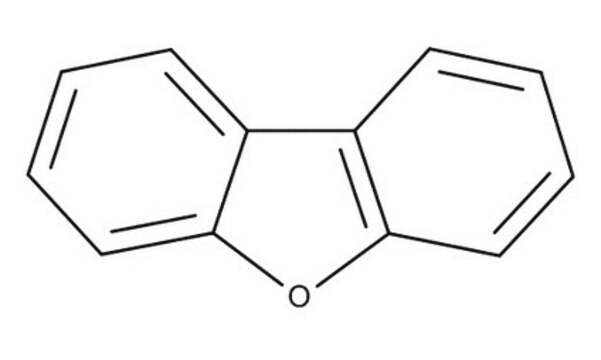
Dibenzothiophene
≥99%
Synonym(s):
DBT, Diphenylene sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H8S
CAS Number:
Molecular Weight:
184.26
Beilstein:
121101
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
crystalline (powder)
color
white
bp
332-333 °C (lit.)
mp
97-100 °C (lit.)
SMILES string
c1ccc2c(c1)sc3ccccc23
InChI
1S/C12H8S/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H
InChI key
IYYZUPMFVPLQIF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dibenzothiophene is an important representative of polycyclic aromatic hydrocarbons (PAHs). Kinetics of hydrodesulfurization of dibenzothiophene on presulflded molybdenaalumina catalyst has been studied in a high-pressure-flow microreactor. Biodesulfurization of dibenzothiophene by selective cleavage of carbon sulphur bonds by a thermophilic bacterium Bacillus subtilis WU-S2B has been reported.
Application
Dibenzothiophene was employed as heavy model sulfur compound to investigate the effect of heavy sulfur compounds on the percentage of sulfur in gasoline range during the Fluid Catalytic Cracking (FCC) process.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
338.0 °F
Flash Point(C)
170 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The effect of heavy aromatic sulfur compounds on sulfur in cracked naphtha.
Valla JA, et al.
Catalysis Today, 127(1), 92-98 (2007)
K Kirimura et al.
Journal of bioscience and bioengineering, 91(3), 262-266 (2005-10-20)
Heterocyclic organosulfur compounds such as dibenzothiophene (DBT) in petroleum cannot be completely removed by hydrodesulfurization using chemical catalysts. A moderately thermophilic bacterium Bacillus subtilis WU-S2B, which could desulfurize DBT at 50 degrees C through the selective cleavage of carbon-sulfur (CS)
Selectivity of molybdenum catalyst in hydrodesulfurization, hydrodenitrogenation, and hydrodeoxygenation: Effect of additives on dibenzothiophene hydrodesulfurization.
Nagai M and Toshiaki Kabe.
J. Catal., 81(2), 440-449 (1983)
Kristina Lång et al.
European radiology, 26(1), 184-190 (2015-05-02)
To assess the performance of one-view digital breast tomosynthesis (DBT) in breast cancer screening. The Malmö Breast Tomosynthesis Screening Trial is a prospective population-based one-arm study with a planned inclusion of 15000 participants; a random sample of women aged 40-74
A Stephen K Hashmi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(21), 6576-6580 (2012-04-21)
With the IPr ligand (IPr=1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene) on gold(I) excellent yields in the benzanellation of 2-substituted thiophenes, benzothiophenes, pyrroles, benzofurans, and indoles were achieved. The 1-siloxybut-3-ynyl side chains, incorporated in the anellation, are easily accessible by the addition of a propargyl metal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service