323373
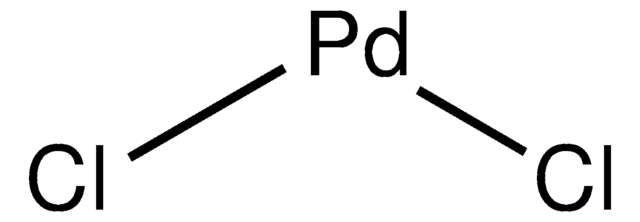
Palladium(II) chloride
99.995%
Synonym(s):
Dichloropalladium, Palladium dichloride, Palladous chloride
About This Item
Recommended Products
Quality Level
Assay
99.995%
form
powder
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
678-680 °C (lit.)
density
4 g/mL at 25 °C (lit.)
SMILES string
Cl[Pd]Cl
InChI
1S/2ClH.Pd/h2*1H;/q;;+2/p-2
InChI key
PIBWKRNGBLPSSY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
Used in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar.
- Carbonylation of ketones to yield diesters.
- Homo-coupling of aryl bromides using ascorbic acid and EDTA..
- Acetylation of alcohols with vinyl acetate.
- Arylation of 2-furaldehyde to yield 5-aryl-2-formylfuran derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Skin Sens. 1
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service