247545
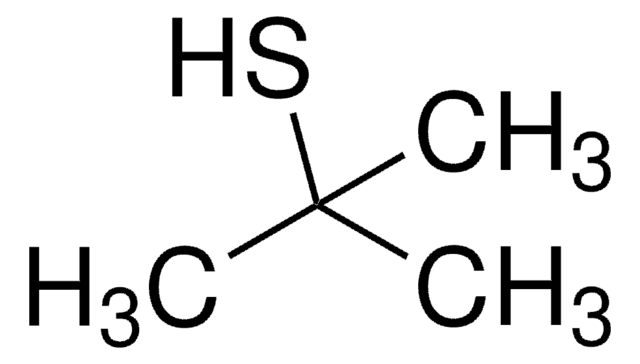
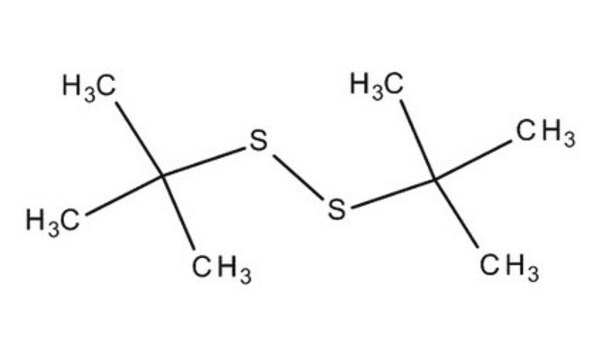
Di-tert-butyl disulfide
97%
Synonym(s):
tert-Butyl disulfide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
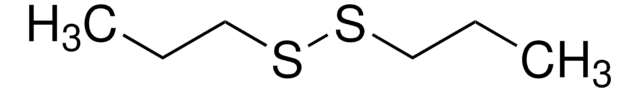
(CH3)3CSSC(CH3)3
CAS Number:
Molecular Weight:
178.36
Beilstein:
1698170
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5 (vs air)
vapor pressure
51.7 mmHg ( 37.7 °C)
Assay
97%
form
liquid
refractive index
n20/D 1.490 (lit.)
bp
200-201 °C (lit.)
density
0.923 g/mL at 25 °C (lit.)
functional group
disulfide
SMILES string
CC(C)(C)SSC(C)(C)C
InChI
1S/C8H18S2/c1-7(2,3)9-10-8(4,5)6/h1-6H3
InChI key
BKCNDTDWDGQHSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Mechanism of the oxidation of di-tert-butyl disulfide in the presence of H2O2 catalyzed by bis(acetylacetonato)oxovanadium and a chiral Schiff-base ligand has been investigated.
Application
Di-tert-butyl disulfide has been used in the preparation of gold-supported thin film from symmetrical and mixed disulphide adsorbates.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Suzanne A Blum et al.
The Journal of organic chemistry, 68(1), 150-155 (2003-01-08)
The mechanism of the oxidation of di-tert-butyl disulfide (1) to the chiral thiosulfinate (2) by H(2)O(2) catalyzed by bis(acetylacetonato)oxovanadium and a chiral Schiff-base ligand (3) has been investigated. Techniques included (51)V NMR spectroscopy, solvent effects on reaction enantioselectivity, and the
Contact angle goniometry, ellipsometry, XPS, and TOF-SIMS analysis of gold-supported, mixed self-assembled monolayers formed from mixed dialkyl disulfides.
Offord DA, et al.
Langmuir, 10(3), 761-766 (1994)
Yang Liu et al.
ACS nano, 12(8), 7803-7811 (2018-07-10)
Heterogeneous copper sulfide based nanostructures have attracted intense attention based on their potential to combine the plasmonic properties of copper-deficient copper sulfides with properties of other semiconductors and metals. In general, copper sulfides are versatile platforms for production of other
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service