All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9NO
CAS Number:
Molecular Weight:
147.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
bp
181-183 °C/24 mmHg (lit.)
mp
69-70 °C (lit.)
solubility
ethanol: 50 mg/mL, clear, faintly yellow
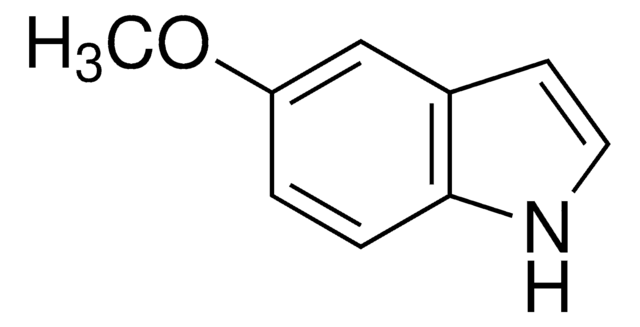
SMILES string
COc1cccc2[nH]ccc12
InChI
1S/C9H9NO/c1-11-9-4-2-3-8-7(9)5-6-10-8/h2-6,10H,1H3
InChI key
LUNOXNMCFPFPMO-UHFFFAOYSA-N
Application
4-Methoxyindole was used for comparing the complexation reaction of β-cyclodextrin (β-CD) with pindolol using reversed-phase liquid chromatography.
Reactant for preparation of:
- GABA analogs
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Anticancer agents
- Integrase strand-transfer inhibitors (INSTIs)
- Inhibitor of Proliferation of Colon Cancer Cells
- Isomeridianin G as GSK-3ß inhibitors
- HIV-1 integrase inhibitors
- Inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carmen Gazpio et al.
Journal of pharmaceutical and biomedical analysis, 37(3), 487-492 (2005-03-03)
The complexation with beta-cyclodextrin (beta-CD) has been investigated using reversed-phase liquid chromatography. The compounds tested have been pindolol and, for comparison purposes, indole and 4-methoxyindole. The retention behaviour has been analysed on a Kromasil 100 C18 column and the mobile
Brenden Barco et al.
Nature communications, 10(1), 3444-3444 (2019-08-03)
Plants synthesize numerous ecologically specialized, lineage-specific metabolites through biosynthetic gene duplication and functional specialization. However, it remains unclear how duplicated genes are wired into existing regulatory networks. We show that the duplicated gene CYP82C2 has been recruited into the WRKY33 regulon
Thomas Heine et al.
Applied biochemistry and biotechnology, 181(4), 1590-1610 (2016-11-11)
The enantioselective epoxidation of styrene and related compounds by two-component styrene monooxygenases (SMOs) has targeted these enzymes for development as biocatalysts. In the present work, we prepare genetically engineered fusion proteins that join the C-terminus of the epoxidase (StyA) to
Jung Min Song et al.
International journal of pharmaceutics, 477(1-2), 96-101 (2014-10-15)
Indole-3-carbinol (I3C), a constituent of commonly consumed Brassica vegetables, has been shown to have anticancer effects in a variety of preclinical models of lung cancer. However, it has shown only limited efficacy in clinical trials, likely due to its poor
Tien-Yuan Wu et al.
Journal of pharmacokinetics and pharmacodynamics, 42(4), 401-408 (2015-07-04)
3,3'-Diindolylmethane (DIM) has been investigated as a potential anti-cancer chemopreventive agent in many preclinical and clinical studies. In this study, we sought to characterize the pharmacokinetics of DIM and to build a pharmacokinetic (PK) and pharmacodynamic (PD) model of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service