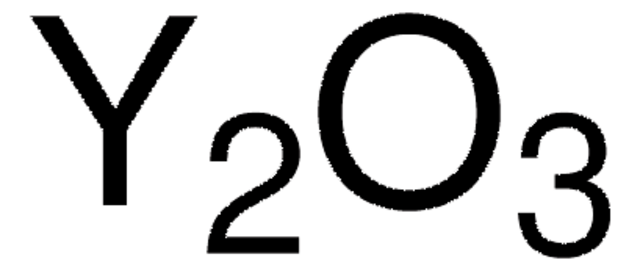205168
Yttrium(III) oxide
99.99% trace metals basis
Synonym(s):
Yttria
About This Item
Recommended Products
Quality Level
Assay
99.99% trace metals basis
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
2410 °C (lit.)
density
5.01 g/mL at 25 °C (lit.)
greener alternative category
SMILES string
O=[Y]O[Y]=O
InChI
1S/3O.2Y
InChI key
SIWVEOZUMHYXCS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Advances in Materials for Solid Oxide Fuel Cells
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
Rechargeable solid-state batteries are becoming increasingly important due to wide-spread use in computers, portable electronics, and vehicular applications.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 205168-10G | 4061838767509 |
| 205168-250G | 4061838767516 |
| 205168-50G | 4061838252371 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service