181285
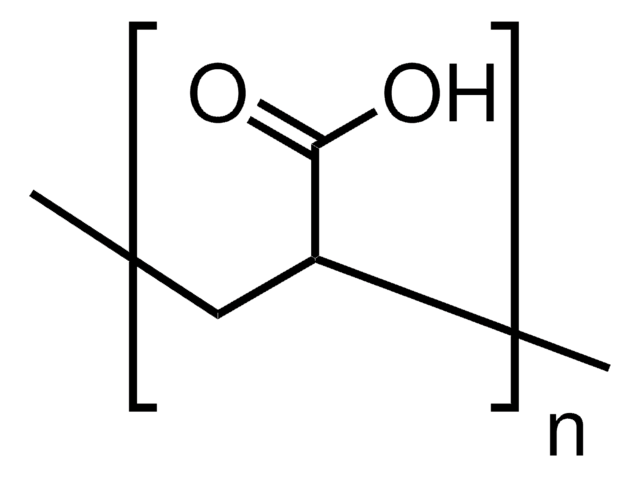
Poly(acrylic acid)
average Mv ~450,000
Synonym(s):
PAA
About This Item
Recommended Products
crosslinking
~0.1 % cross-linked
mol wt
average Mv ~450,000
viscosity
350-2500 cP
transition temp
Tg 106 °C
InChI
1S/C3H4O2.Na/c1-2-3(4)5;/h2H,1H2,(H,4,5);/q;+1/p-1
InChI key
NNMHYFLPFNGQFZ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other applications may include:
- to study solute diffusion in Polyvinyl alcohol/PAA copolymer hydrogel
- synthesizing poly(N-isopropylacrylamide)-block-PAA copolymer which responds to both temperature and pH stimuli
- in preparing block copolymer of oligo (methyl methacrylate)/PAA for micellar delivery of hydrophobic drugs
- as thickening agent for adhesives.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
New methods for materials fabrication at the micro- and nanoscale will drive scientific and technological advances in areas of materials science, chemistry, physics, and biology. The broad diversity of potentially relevant materials, length scales, and architectures underscores the need for flexible patterning approaches. One important example is the fabrication of 3D periodic structures composed of colloidal, polymeric, or semiconductor5 materials.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service