All Photos(1)
About This Item
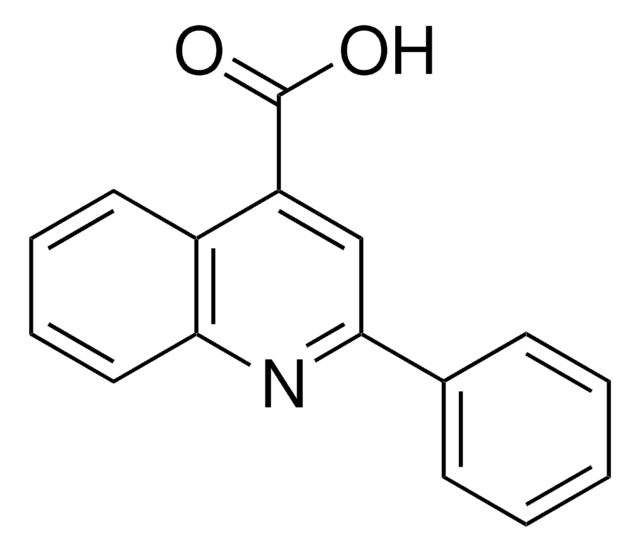
Empirical Formula (Hill Notation):
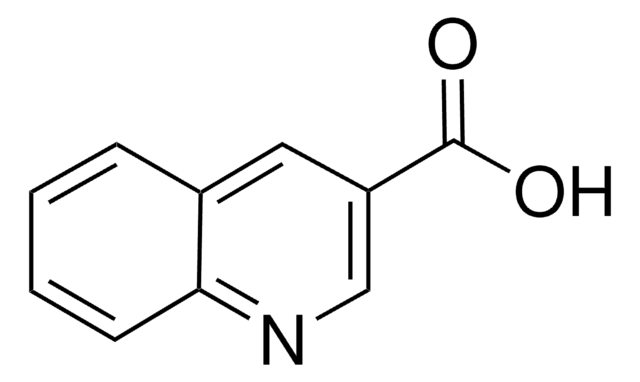
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
5224
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
254-255 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
InChI key
VQMSRUREDGBWKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
Biochem/physiol Actions
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D L Dexter et al.
Cancer research, 45(11 Pt 1), 5563-5568 (1985-11-01)
A novel, substituted 4-quinolinecarboxylic acid (NSC 339768) demonstrated antitumor activity against L1210 leukemia and B16 melanoma in the National Cancer Institute's Developmental Therapeutics Program. An extensive analogue synthesis program was initiated; over 200 derivatives were synthesized and tested for anticancer
[A case of Gaucher's disease treated with hydroxyphenylcinchoninic acid].
P DANIEL MARTINEZ et al.
Boletin medico del Hospital Infantil de Mexico, 8(2), 189-194 (1951-04-01)
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 55 ( Pt 7), 1192-1195 (1999-08-13)
The previously undescribed title substance, C10H7NO2.-3H2O, crystallized in the centrosymmetric space group P1 with one zwitterionic organic molecule and three water molecules in the asymmetric unit. One N-H...O and six O-H...O hydrogen bonds are present in this structure, with donor-acceptor
Murugesan Dinakaran et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 4(5), 482-491 (2008-09-11)
Thirty four novel 7-fluoro/nitro-1,2-dihydro-5-oxo-8-(sub)-5H-thiazolo[3,2-a]quinoline-4-carboxylic acids were synthesized from 2,4-dichlorobenzoic acid and 2,4-dichloro-5-fluoroacetophenone by multi step reaction, evaluated for in vitro and in vivo antimycobacterial activities against Mycobacterium tuberculosis H37Rv (MTB), multi-drug resistant Mycobacterium tuberculosis (MDR-TB) and Mycobacterium smegmatis (MC2) and
He Huang et al.
The Journal of organic chemistry, 74(15), 5476-5480 (2009-07-04)
We developed a simple and convenient copper-catalyzed method for the synthesis of quinoline-2-carboxylate derivatives through sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The efficiency of this system allowed the reactions to be carried
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service