163171
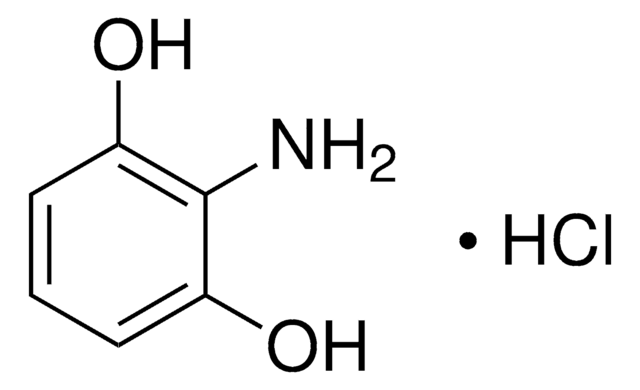
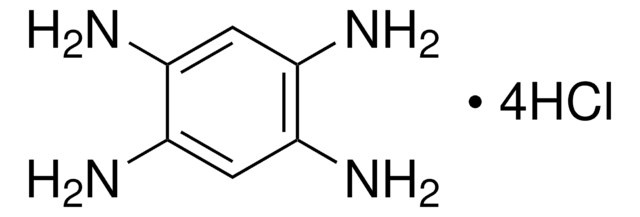
4-Aminoresorcinol hydrochloride
96%
Synonym(s):
2,4-Dihydroxyaniline hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
H2NC6H3-1,3-(OH)2 · HCl
CAS Number:
Molecular Weight:
161.59
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
powder
mp
220 °C (dec.) (lit.)
SMILES string
Cl.Nc1ccc(O)cc1O
InChI
1S/C6H7NO2.ClH/c7-5-2-1-4(8)3-6(5)9;/h1-3,8-9H,7H2;1H
InChI key
LLJMPRKYLLJAEB-UHFFFAOYSA-N
Related Categories
General description
Electrochemical oxidation of 4-aminoresorcinol hydrochloride (2,4-dihydroxyaniline hydrochloride) has been studied using a boron-doped diamond electrode as anode. It is an inhibitor of meta-cleavage dioxygenase enzyme isolated from cell extracts of Bordetella sp. strain 10d.
Application
4-Aminoresorcinol hydrochloride was used in an enzyme assay during isolation of soil bacterium Bordetella sp. strain 10d.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shinji Takenaka et al.
European journal of biochemistry, 269(23), 5871-5877 (2002-11-26)
A bacterial strain that grew on 4-amino-3-hydroxybenzoic acid was isolated from farm soil. The isolate, strain 10d, was identified as a species of Bordetella. Cell extracts of Bordetella sp. strain 10d grown on 4-amino-3-hydroxybenzoic acid contained an enzyme that cleaved
M J Pacheco et al.
Journal of hazardous materials, 186(2-3), 1033-1041 (2010-12-21)
The electrochemical oxidation of four aromatic amines, with different substituent groups, 3-amino-4-hydroxy-5-nitrobenzenesulfonic acid (A1), 5-amino-2-methoxybenzenesulfonic acid (A2), 2,4-dihydroxyaniline hydrochloride (A3) and benzene-1,4-diamine (A4), was performed using as anode a boron-doped diamond electrode, commercially available at Adamant Technologies. Tests were run
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service