158186
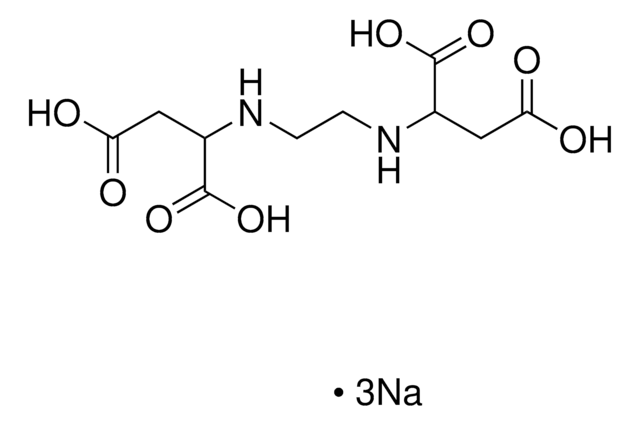
Ethylenediamine-N,N′-diacetic acid
≥98%
Synonym(s):
EDDA, N,N′-Ethylenediglycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
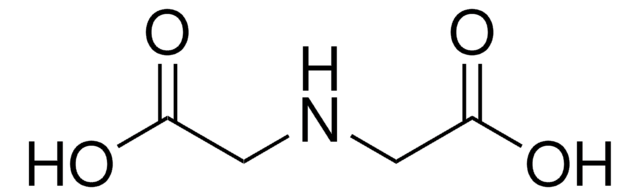
HOOCCH2NHCH2CH2NHCH2COOH
CAS Number:
Molecular Weight:
176.17
Beilstein:
1778355
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
mp
224 °C (dec.) (lit.)
functional group
amine
carboxylic acid
SMILES string
OC(=O)CNCCNCC(O)=O
InChI
1S/C6H12N2O4/c9-5(10)3-7-1-2-8-4-6(11)12/h7-8H,1-4H2,(H,9,10)(H,11,12)
InChI key
IFQUWYZCAGRUJN-UHFFFAOYSA-N
Application
Ethylenediamine-N,N′-diacetic acid (EDDA) is a chelating agent that can be used to synthesize:
- Binary and ternary copper(II) complexes with potent proteasome inhibitory properties.
- Pd(EDDA) complexes which can coordinate with amino acids, peptides, or DNA units.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Clara L Santos-Cuevas et al.
Nuclear medicine communications, 29(8), 741-747 (2008-08-30)
The gastrin-releasing peptide receptor (GRP-R) is expressed in several normal human tissues and is overexpressed in various human tumors including breast, prostate, small-cell lung cancer and pancreatic cancer. Recently, 99mTc-EDDA/HYNIC-[Lys]-bombesin (99mTc-HYNIC-BN) was reported as a radiopharmaceutical with high stability in
Olivier Dalmas et al.
Structure (London, England : 1993), 18(7), 868-878 (2010-07-20)
The transmembrane conformation of Thermotoga maritima CorA, a magnesium transport system, has been studied in its Mg(2+)-bound form by site-directed spin labeling and electron paramagnetic resonance spectroscopy. Probe mobility together with accessibility data were used to evaluate the overall dynamics
Alma D Miranda-Olvera et al.
Bioconjugate chemistry, 18(5), 1560-1567 (2007-08-02)
Two synthetic procedures for HYNIC oxytocin labeling were developed: one based on an orthogonal protection approach and the other with prelabeled (Boc)HYNIC-(Fmoc) amino acids. Both procedures were compared and applied to the preparation of several HYNIC-oxytocin derivatives where ligand position
Jelena M Vujić et al.
European journal of medicinal chemistry, 45(9), 3601-3606 (2010-06-24)
Four novel bidentate N,N'-ligand precursors, including O,O'-dialkyl esters (alkyl = ethyl, n-propyl, n-butyl and n-pentyl), L1 x 2 HCl-L4 x 2 HCl, of (S,S)-ethylenediamine-N,N'-di-2-(4-methyl)-pentanoic acid dihydrochloride [(S,S)-H(4)eddl]Cl(2) and the corresponding palladium(II) complexes 1-4, were prepared and characterized by IR, (1)H
Levente K Meszaros et al.
Dalton transactions (Cambridge, England : 2003), 40(23), 6260-6267 (2011-02-26)
6-Hydrazinonicotinic acid (HYNIC, 1) is a well-established bifunctional technetium-binding ligand often used to synthesise bioconjugates for radiolabelling with Tc-99m. It is capable of efficient capture of technetium at extremely low concentrations, but the structure of the labelled complexes is heterogeneous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service