All Photos(2)
About This Item
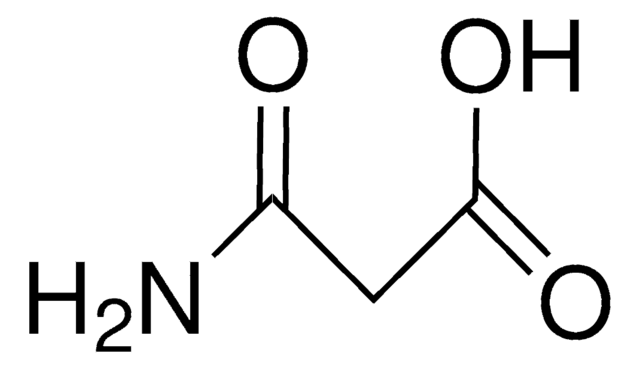
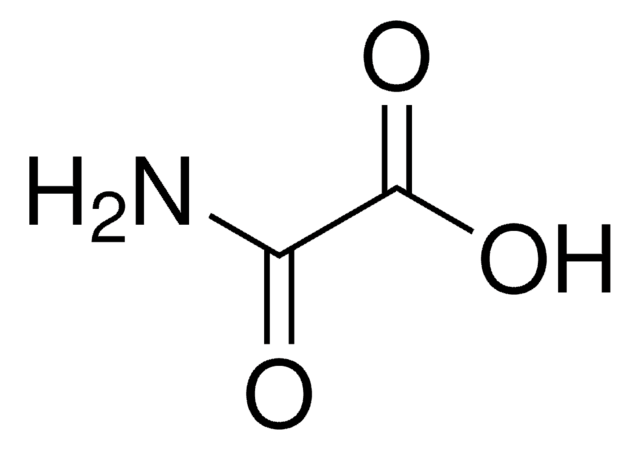
Linear Formula:
CH2(CONH2)2
CAS Number:
Molecular Weight:
102.09
Beilstein:
1751401
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
172-175 °C (lit.)
fluorescence
λex 367 nm; λem 445 nm (α-keto acid adduct)
SMILES string
NC(=O)CC(N)=O
InChI
1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChI key
WRIRWRKPLXCTFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Application
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alok Rout et al.
Dalton transactions (Cambridge, England : 2003), 43(4), 1862-1872 (2013-11-22)
A new non-fluorinated malonamide-based ionic liquid extractant was synthesized and investigated for the extraction behavior of europium(III) and other trivalent rare-earth ions from nitric acid medium. The extractant was the functionalized ionic liquid trihexyl(tetradecyl)phosphonium N,N,N',N'-tetra(2-ethylhexyl)malonate, [P66614][MA], and it was used
Ranjeet A Dhokale et al.
Organic letters, 14(15), 3994-3997 (2012-07-27)
A facile, fluoride-induced transition-metal-free chemoselective α-arylation of β-dicarbonyl compounds (malonamide esters) at room temperature using aryne intermediates has been demonstrated. Selective mono- or diarylation and generation of a quaternary benzylic stereocenter have also been achieved. The methodology will be highly
Suban K Sahoo et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 63(3), 574-586 (2005-07-19)
A new dipodal ligand, N,N'-bis{2-[(2-hydroxybenzylidine)amino]ethyl}malonamide (BHAEM) was synthesized by Schiff base condensation of N,N'-bis(2-aminoethyl)malonamide with two equivalent of salicylaldehyde and characterized on the basis of elemental analyses and various spectral (UV-vis, IR, (1)H NMR and (13)C NMR) data. The complexation
Amélie Banc et al.
The journal of physical chemistry. B, 115(6), 1376-1384 (2011-01-22)
In this paper we used a surfactant-stabilized lyotropic lamellar model system to study the interfacial behavior of an ion-extracting agent: N(1),N(3) dimethyl-N(1),N(3)-dibutyl-2-tetradecylmalonamide (DMDBTDMA). An analysis of small-angle X-ray scattering (SAXS) and polarized attenuated total reflectance-Fourier transform infrared (ATR-FTIR) data enabled
Mi-Hyun Kim et al.
Organic letters, 12(12), 2826-2829 (2010-05-27)
A new enantioselective synthetic method of (-)-paroxetine is reported. (-)-Paroxetine could be obtained in 15 steps (95% ee and 9.1% overall yield) from N,N-bis(p-methoxyphenyl)malonamide tert-butyl ester via the enantioselective phase-transfer catalytic alkylation and the diastereoselective Michael addition as the key
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service