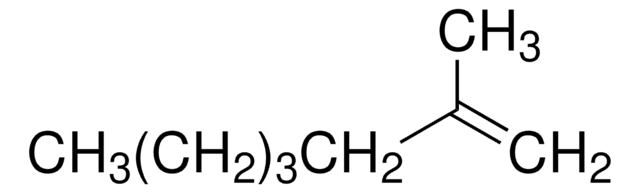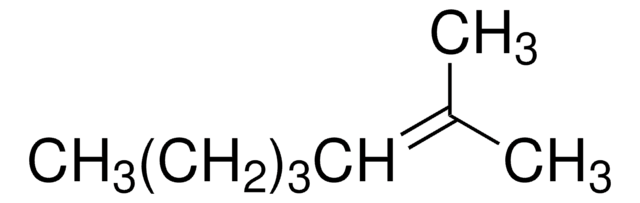All Photos(1)
About This Item
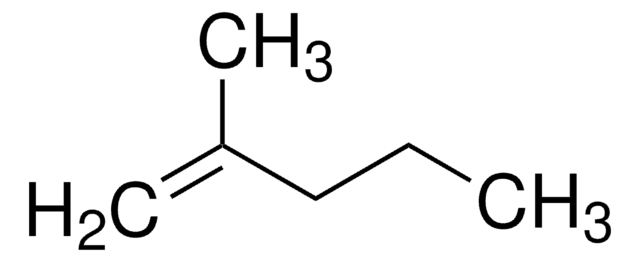
Linear Formula:
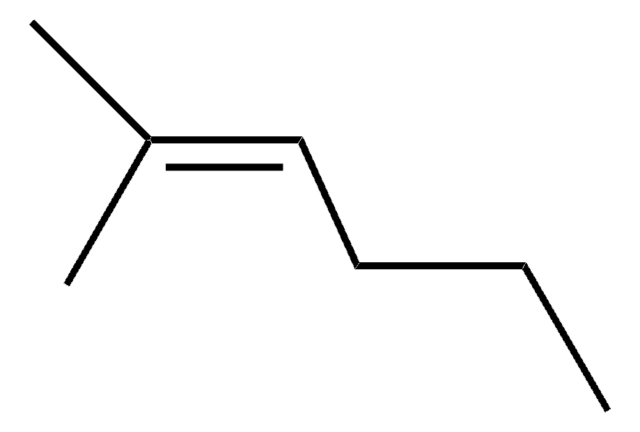
CH3(CH2)3C(CH3)=CH2
CAS Number:
Molecular Weight:
98.19
Beilstein:
1732738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1 (vs air)
Assay
96%
refractive index
n20/D 1.403 (lit.)
bp
92 °C (lit.)
density
0.697 g/mL at 25 °C (lit.)
SMILES string
CCCCC(C)=C
InChI
1S/C7H14/c1-4-5-6-7(2)3/h2,4-6H2,1,3H3
InChI key
IRUDSQHLKGNCGF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Methyl-1-hexene is a less reactive olefin that is hydroformylated in the presence of phosphite-modified rhodium catalysts. The epoxidation of 2-methyl-1-hexene is catalyzed by human and rat P450 enzymes.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
17.6 °F - closed cup
Flash Point(C)
-8 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Chiappe et al.
Chemical research in toxicology, 11(12), 1487-1493 (1998-12-22)
The epoxidation of 1-hexene (1a) and 2-methyl-1-hexene (1b), two hydrocarbons present in the ambient air as pollutants, is catalyzed by some human and rat P450 enzymes. The enantioselectivities of these processes, when the reactions were carried out using rat and
Hydroformylation of less reactive olefins with modified rhodium catalysts.
Van Leeuwen PWNM and Roobeek CF.
Journal of Organometallic Chemistry, 258(3), 343-350 (1983)
Hiroshi Toda et al.
Applied and environmental microbiology, 81(6), 1919-1925 (2015-01-04)
We describe the development of biocatalysis for producing optically pure straight-chain (S)-epoxyalkanes using styrene monooxygenase of Rhodococcus sp. strain ST-10 (RhSMO). RhSMO was expressed in the organic solvent-tolerant microorganism Kocuria rhizophila DC2201, and the bioconversion reaction was performed in an
Protocols
GC Analysis of Hydrocarbons in Gasoline on Petrocol® DH, Isothermal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service