L-Glutamine in Cell Culture
Why is L-glutamine important in cell culture?
L-glutamine is a common a serum-free medium supplement used in biomanufacturing applications, tissue engineering, specialty cell cultures like hybridoma culture, and other common mammalian cell culture applications. However, because l-glutamine is an unstable essential amino acid, most commercially available media are formulated with free L-glutamine included in the basal formula or added to liquid formulations at time of use.
We offer glutamine for cell culture as a dipeptide or a protein hydrolysate. Some proprietary media used in biomanufacturing are supplemented with L-glutamine in dipeptide forms, such as alanyl-l-glutamine and glycyl-l-glutamine. A less well-defined source of L-glutamine comes from the use of protein hydrolysates, especially gluten hydrolysates. For serum-free or animal-protein-free media needs, such as culturing recombinant Chinese Hamster Ovary cells (rCHO), wheat gluten is a rich source of peptidyl glutamine.
As a supplement in classical cell culture media, the concentration of L-glutamine used ranges from 0.5 mM in Ames' Medium to 10 mM in MCDB Media 131. The more typical concentrations in those media for biomanufacturing and tissue engineering applications is between 2 and 4 mM.
The optimal L-glutamine concentration in DMEM/F12 Nutrient Mixture is 2.5 mM, while in Serum-Free/Protein Free Hybridoma Medium is it is 2.7 mM. For DMEM, GMEM, IMDM and H-Y medium, the optimal concentration is 4 mM. IMDM is often used as a starting formulation for proprietary hybridoma cell culture media; hybridoma cells grow better in concentrations of L-glutamine that are above the average levels found in media.
L-glutamine in Cell Culture Systems
Because of its chemical instability and importance for cell growth and function, it is critical that the delivery of L-glutamine is optimized to each unique cell culture process. Glutamine has the molecular formula of C5H10N2O3 and the molecular weight of 146.15g/mol, with an isoelectric point of 5.65 and a pka of 2.17 and 9.13. Understanding this chemistry and the multiple delivery forms of L-glutamine and its alternatives is required for effectively using the supplement in cell culture applications.
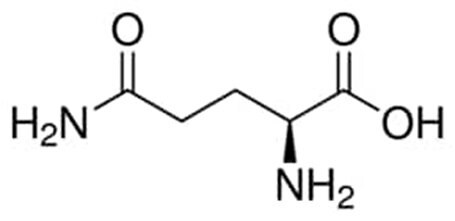
Figure 1.L-glutamine chemical structure.
Glutamine supports the growth of cells that have high energy demands and synthesize large amounts of proteins and nucleic acids. It is also an alternative energy source for rapidly dividing cells and cells that use glucose inefficiently. Cells require the nitrogen atoms in glutamine to build molecules such as nucleotides, amino acids, amino-sugars, and vitamins.
Glutamine is a precursor of glutamate, a key amino acid used for the transamination of alpha ketoacids to form other alpha amino acids. It contains one atom of nitrogen as an amide and another atom of nitrogen as an amine; it also transports and delivers nitrogen to cells in quantities that are not as toxic as free ammonium.
When glucose levels are low and energy demands are high, cells can metabolize amino acids for energy. The glutamine amide nitrogen is used in the synthesis of the vitamins NAD and NADP, purine nucleotides, CTP from UTP and asparagine. Nitrogen initially stored in glutamine can also be used to produce carbamyl phosphate for the synthesis of pyrimidines. Glutamine is one of the most readily available amino acids for use as an energy source and it is a major source of energy for many rapidly dividing cell types in vitro.
Glutamine stability in cell culture media
When glutamine is present as an amino acid residue in proteins or peptides, it is stable. The conditionally essential amino acid is a freely soluble neutral amino acid containing an R-group amide. As it is unstable, it can break down non-enzymatically into ammonia and pyroglutamate (pyrrolidonecarboxylic acid) in liquid media. The breakdown of L-glutamine over time is dependent on pH, temperature, and the presence of various anions.
Glutamine deamination, which is a reaction that removes the amino group, occurs in both acidic and basic conditions. The reaction occurs significantly more rapidly in the presence of phosphate or bicarbonate in the media. At a fixed phosphate concentration in the liquid media, the deamination rate increases as the pH increases from 4.3 to 10.
L-glutamine biochemistry
Reactions that fix nitrogen into glutamate and glutamine consume energy equivalents, such as NADH, NADPH, or ATP. Glutamate is synthesized from ammonium and alpha ketoglutaric acid, a tricarboxylic acid (TCA) cycle intermediate. This synthesis requires the oxidation of either NADH or NADPH. The enzymes involved in glutamate synthesis, glutamate dehydrogenase (EC 1.4.1.4) and glutamate synthase (EC 1.4.1.13), are reversible.
Ammonium, an inorganic source of nitrogen used by cells, is initially incorporated into organic nitrogen as an amine of glutamate or an amide of glutamine. These two amino acids provide the primary reservoirs of nitrogen for the synthesis of proteins, nucleic acids, and other nitrogenous compounds. Ammonium produced in vivo, but not in vitro, can be metabolized to urea. Under some in vitro conditions, ammonia accumulates in the extracellular medium as ammonium ion.
Glutamine is synthesized from ammonium and glutamate molecules. This synthesis consumes energy in the form of ATP. The enzyme responsible for this synthesis, glutamine synthetase (EC 6.3.1.2), is highly regulated to limit the production of glutamine to cell requirements. The catabolism of glutamine to glutamate and ammonium is mediated by mitochondrial enzymes called glutaminases (EC 3.5.1.2).
To continue reading please sign in or create an account.
Don't Have An Account?