Protocol Guide: Exosome Labeling Using PKH Lipophilic Membrane Dyes
Exosomes are microscale extracellular vesicles that are present in most somatic fluids including blood and urine, as well as the media of cell cultures. These-lipid-bearing microvesicles range in size from 30 to 100 nm. As they often contain key biomolecules like proteins and nucleic acids (both DNA and RNA) derived from their cell of origin, exosomes have generated interest for their potential as targets of cancer therapeutics. Recent studies support that exosomes are involved with intercellular communication, signaling, and the immune response, as well as management of cellular waste.
PKH and CellVue® Fluorescent Cell Linker Kits provide fluorescent labeling of live cells over extended periods, with no apparent toxic effects. These cell linkers use selective partitioning to incorporate aliphatic reporter molecules into the lipid bilayer of the plasma membrane. The membrane dye PKH67 is a green fluorochrome with excitation (490 nm) and emission (504 nm) similar to fluorescein and FITC. PKH26 is a fluorochrome in the red spectrum with peak excitation (551 nm) and emission (567 nm) characteristics compatible with rhodamine or phycoerythrin (PE) detection channels, and may also be excited by a 488 nm laser. CellVue® Claret is a far-red fluorochrome which is unaffected by pH in physiologic ranges, and has excitation (655 nm) and emission (675 nm) characteristics compatible with a far red laser.
PKH dyes have been successfully used to label exosomes and extracellular vesicles for in vitro and in vivo tracking experiments. The exosome labeling protocol below details steps for reliable fluorescent labeling of exosomes using PKH dyes for microvesicle tracking experiments.
Exosome Labeling Protocol
- Start with freshly isolated exosome pellets prepared from ultracentrifugation or microfiltration. Exosome Isolation Protocol
- Cool the ultracentrifuge to 2-8 °C.
- Combine pellets from multiple tubes into one tube for each sample, and measure total volume.
- Bring the volume of the pellet sample up to 1mL using Diluent C from the PKH67 (PKH67GL, MINI67, MIDI67) or PKH26 (PKH26GL, MINI26, MIDI26) kit for each sample.
- Determine the pellet sample with the largest volume, and add that volume of exosome-free media to a new ultracentrifuge tube. Bring volume to 1mL with Diluent C.
- Add 6uL PKH67 or PKH26 dye into each of the 1mL Diluent C tubes from steps 4-5.
- Mix continuously for 30 seconds by gentle pipetting. Let stand at room temperature for 5 minutes.
- Quench by adding 2mL 10% BSA (A9418) in PBS (D8537). Bring the volume up to 8.5 mL in serum-free media.
- Make a 0.971 M sucrose solution (S1888).
- Add 1.5mL of the sucrose solution by pipetting slowly and carefully into the bottom of your tube, making sure not to create turbulence. The exosomes-PKH67 solution will remain on top of a sucrose cushion.
- Centrifuge at 190,000 G for 2 hours at 2-8 °C.
Note: Exosomes will be in the pellet, and most of the excess dye should be in the interface layer. - Carefully aspirate the media and interface layer.
- Resuspend the exosome pellet in 1X PBS (D8537) by gentle pipetting.
- Transfer to a Amicon 10 kDa MWCO (molecular weight cutoff) filter column. Add 9mL PBS, 0.75 mL media.
- Spin at 3000 x g in the high-speed centrifuge for 40 minutes, and perform as many shorter spins as needed to reduce volume to 0.5-1 mL.
- Recover concentrate from Amicon column, store in microfuge tube on ice. Use labeled exosomes a soon as possible to ensure highest possible fluorescent intensity.
Analysis of Labeled Exosomes Using Amnis® Imaging Flow Cytometry

Figure 1. Cellular uptake of exosomes. Exosomes from SW780 cells were isolated and labeled with PKH26. The labeled exosomes were cultured with SW780 for 17 hours and analyzed using an Amnis® ImageStream flow cytometry instrument. PKH26 bright spots were seen in the cells cultured with loaded exosomes, and not seen in control cells.
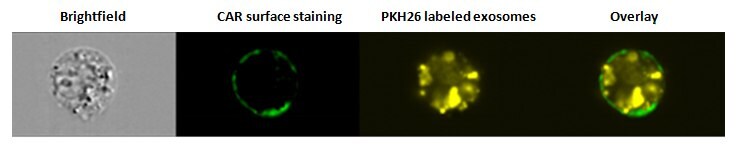
Figure 2. Internalization of exosomes. SW780 cells were stained with the surface marker CAR (Coxsackie and Adenovirus Receptor) after PKH26 labeled exosome uptake and analyzed using an Amnis® ImageStream flow cytometer. Overlay image demonstrates that exosomes have been internalized.

Figure 3. Exosome storage stability. Exosomes were isolated and stained with PKH26. The PKH26-labeled exosomes were either added to SW780 cultures immediately, or stored overnight at 2-8 °C or-20 °C. After the overnight storage, the exosomes were added to the SW780 cultures and analyzed using an Amnis® ImageStream flow cytometry instrument. In all samples, uptake was measured after 4 hours in culture. Exosome uptake can be detected in cultures using stored exosomes, but some loss of fluorescence intensity may be observed.

Figure 4. Exosome uptake specificity. SW780 cells were cultured with PKH labeled exosomes and collected for analysis. A sample was treated with trypsin to determine if this would strip the exosomes. A sample was cultured at 2-8 °C and another culture had sodium azide added to the culture. Exosome detection is resistant to trypsin. However, no exosomes uptake was present in either the 2-8 °C culture or the sodium azide-treated culture.
Data courtesy of Patricia Simms, Core Facility Manager, Flow Cytometry Core Facility, Loyola University, Chicago
CellVue® is a registered trademark of PTI Research, Inc
Amnis®, FlowSight®, ImageStream®, Guava® and Muse® are registered trademarks of Merck KGaA, Darmstadt, Germany
Duolink® is a registered trademark of Sigma-Aldrich Co
To continue reading please sign in or create an account.
Don't Have An Account?