55276-U
HybridSPE®-Phospholipid solid phase extraction (SPE) Cartridge
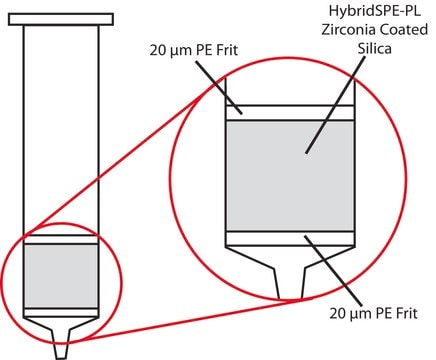
Cartridge, bed wt. 30 mg, volume 1 mL, pk of 200, polypropylene material (hardware), PE frit (20 μm)
Synonym(s):
HybridSPE (phospholipid and protein removal) SPE cartridge tube, 6 mL
About This Item
Recommended Products
product name
HybridSPE®-Phospholipid, Cartridge, bed wt. 30 mg, volume 1 mL, pk of 200, polypropylene material (hardware), PE frit (20 μm)
material
PE frit (20 μm)
polypropylene hardware
composition
bed wt., 30 mg
packaging
pk of 200
technique(s)
solid phase extraction (SPE): suitable
volume
1 mL
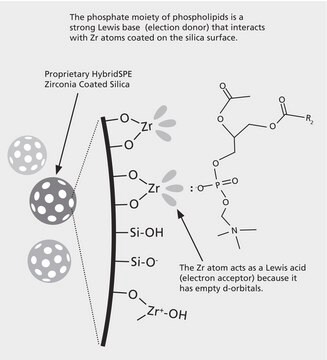
matrix active group
zirconia-based phase
Looking for similar products? Visit Product Comparison Guide
General description
The "In-well" and "In-cartridge" precipitation methods are available for the HybridSPE-Phospholipid 96-well version and HybridSPE-Phospholipid Ultra cartridge in which biological plasma/serum is first added to either the well or cartridge, followed by acidified acetonitrile (precipitation agent). After a brief mixing/vortexing step, vacuum is applied. Because the 96-well and Ultra cartridge versions contain a series of low porosity hydrophobic filters/frits, the packed-bed filter/frit assembly acts as a depth filter facilitating the concurrent removal of both phospholipids and precipitated proteins during the extraction process. Standard HybridSPE-Phospholipid cartridges require an "off-line" precipitation method.
Application
- Less is more: a methodological assessment of extraction techniques for per- and polyfluoroalkyl substances (PFAS) analysis in mammalian tissues.: This study evaluates various extraction techniques, including the use of HybridSPE-Phospholipid, for analyzing PFAS in mammalian tissues. The research highlights the importance of effective phospholipid removal to ensure accurate PFAS quantification (Mertens et al., 2023).
- Rapid analysis of 65 pharmaceuticals and 7 personal care products in plasma and whole-body tissue samples of fish using acidic extraction, zirconia-coated silica cleanup, and liquid chromatography-tandem mass spectrometry.: The study includes HybridSPE-Phospholipid as part of the cleanup process, demonstrating its efficiency in removing phospholipids to improve the detection of pharmaceuticals and personal care products in biological samples (Tanoue et al., 2020).
- Multi LC-MS/MS and LC-HRMS Methods for Determination of 24 Mycotoxins including Major Phase I and II Biomarker Metabolites in Biological Matrices from Pigs and Broiler Chickens.: This research utilizes HybridSPE-Phospholipid for the cleanup of biological matrices, enhancing the accuracy of mycotoxin detection by effectively removing interfering phospholipids (Lauwers et al., 2019).
- Effective phospholipid removal from plasma samples by solid phase extraction with the use of copper (II) modified silica gel cartridges.: The study compares various phospholipid removal techniques, highlighting the performance of HybridSPE-Phospholipid in achieving high phospholipid removal efficiency from plasma samples (Flieger et al., 2017).
- Liquid chromatography mass spectrometry determination of perfluoroalkyl acids in environmental solid extracts after phospholipid removal and on-line turbulent flow chromatography purification.: The research demonstrates the application of HybridSPE-Phospholipid in the removal of phospholipids from environmental solid extracts, facilitating the accurate measurement of perfluoroalkyl acids (Mazzoni et al., 2016).
Features and Benefits
- Merges the simplicity of protein precipitation and the selectivity of SPE via the targeted removal of phospholipids
- Reduce ion-suppression through the complete removal of phospholipids and precipitated proteins
- 2-3 step generic procedure
- Minimal to no method development
- Available in 96-well and 1 mL cartridge dimensions
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
We are presenting an article focusing on ion-suppression and phospholipid contamination and some of their major causes and difficulties.
Protocols
A simple method to enrich phospholipids from plasma samples, involving a HybridSPE-PPT 96-well plate that both retains phospholipids and removes precipitated proteins.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service