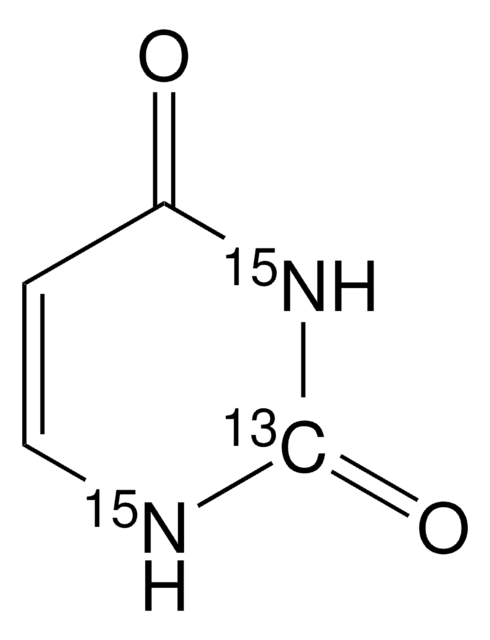
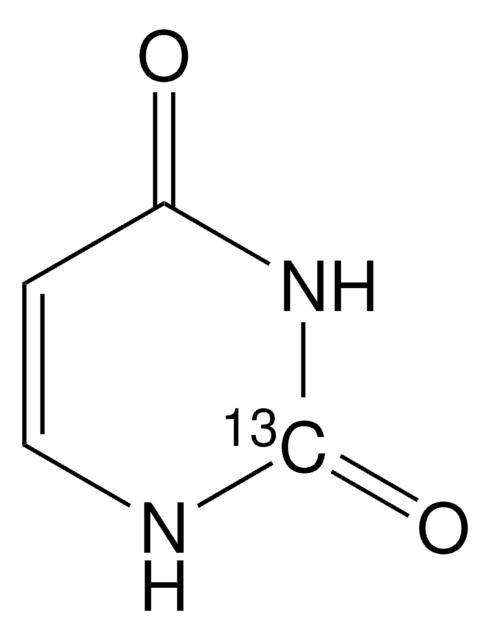
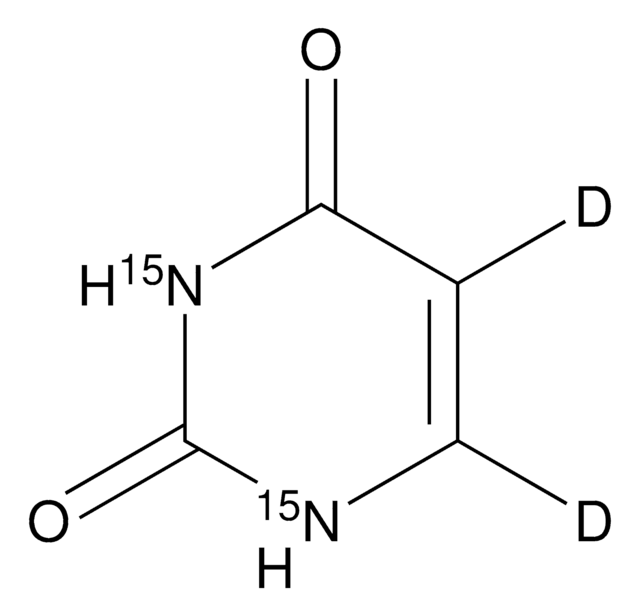
U0750
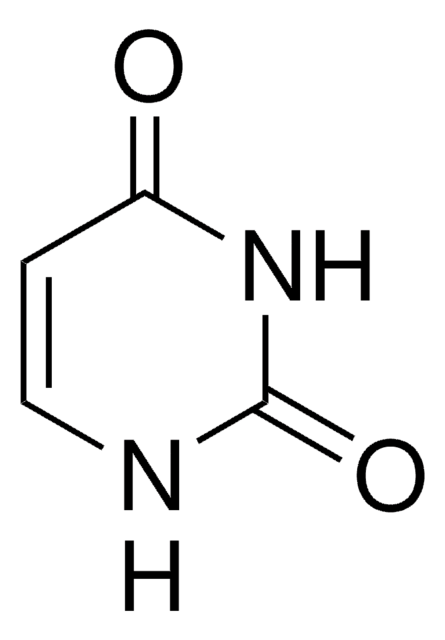
Uracil
≥99.0%
Synonym(s):
2,4(1H,3H)-Pyrimidinedione, 2,4-Dihydroxypyrimidine, 2,4-Pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(6)
About This Item
Empirical Formula (Hill Notation):
C4H4N2O2
CAS Number:
Molecular Weight:
112.09
Beilstein:
507828
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Quality Level
Assay
≥99.0%
form
powder
mp
>300 °C (lit.)
SMILES string
O=C1NC=CC(=O)N1
InChI
1S/C4H4N2O2/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
InChI key
ISAKRJDGNUQOIC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Uracil belongs to the pyrimidine nucleobase family. It is naturally occurring and is an important and active part of RNA.
Application
Uracil has been used as:
- a nucleotide standard to determine DNA methylation by high-performance liquid chromatography
- a component of synthetic define medium for culturing Saccharomyces cerevisiae and its strain
- a component of the amino acid supplement to culture Bacillus sp
Biochem/physiol Actions
Uracil and its derivatives play an integral part in medicinal chemistry for the synthesis of cancer, viral infections, autosomal recessive disorder, thyroid, and diabetic drugs.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sandra M Carvalho et al.
PloS one, 8(3), e58492-e58492 (2013-03-19)
Links between carbohydrate metabolism and virulence in Streptococcus pneumoniae have been recurrently established. To investigate these links further we developed a chemically defined medium (CDM) and standardized growth conditions that allowed for high growth yields of the related pneumococcal strains
William B White et al.
The New England journal of medicine, 369(14), 1327-1335 (2013-09-03)
To assess potentially elevated cardiovascular risk related to new antihyperglycemic drugs in patients with type 2 diabetes, regulatory agencies require a comprehensive evaluation of the cardiovascular safety profile of new antidiabetic therapies. We assessed cardiovascular outcomes with alogliptin, a new
Daisuke Katagiri et al.
Journal of the American Society of Nephrology : JASN, 24(12), 2034-2043 (2013-10-05)
Accumulating evidence of the beyond-glucose lowering effects of a gut-released hormone, glucagon-like peptide-1 (GLP-1), has been reported in the context of remote organ connections of the cardiovascular system. Specifically, GLP-1 appears to prevent apoptosis, and inhibition of dipeptidyl peptidase-4 (DPP-4)
Jaunius Urbonavicius et al.
Methods in enzymology, 425, 103-119 (2007-08-04)
Formation of 5-methyluridine (ribothymidine) at position 54 of the T-psi loop of tRNA is catalyzed by site-specific tRNA methyltransferases (tRNA[uracil-54,C5]-MTases). In eukaryotes and many bacteria, the methyl donor for this reaction is generally S-adenosyl-L-methionine (S-AdoMet). However, in other bacteria, like
Mirta M L Sousa et al.
Molecular aspects of medicine, 28(3-4), 276-306 (2007-06-26)
Uracil is usually an inappropriate base in DNA, but it is also a normal intermediate during somatic hypermutation (SHM) and class switch recombination (CSR) in adaptive immunity. In addition, uracil is introduced into retroviral DNA by the host as part
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service