N3510
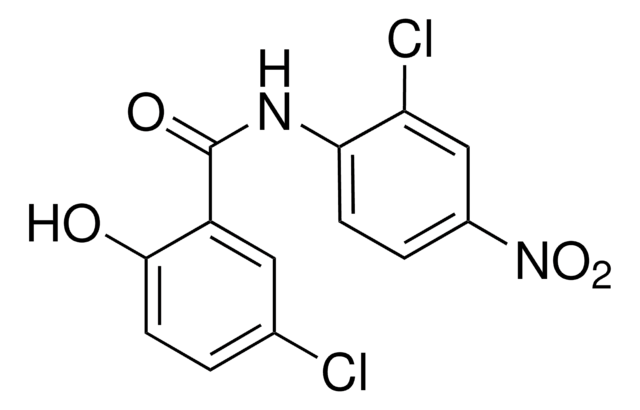
Niclosamide
Synonym(s):
2′,5-Dichloro-4′-nitrosalicylanilide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H8Cl2N2O4
CAS Number:
Molecular Weight:
327.12
Beilstein:
2820605
EC Number:
MDL number:
UNSPSC Code:
51102829
PubChem Substance ID:
NACRES:
NA.85
Recommended Products
form
powder
Quality Level
antibiotic activity spectrum
parasites
Mode of action
enzyme | inhibits
SMILES string
Oc1ccc(Cl)cc1C(=O)Nc2ccc(cc2Cl)[N+]([O-])=O
InChI
1S/C13H8Cl2N2O4/c14-7-1-4-12(18)9(5-7)13(19)16-11-3-2-8(17(20)21)6-10(11)15/h1-6,18H,(H,16,19)
InChI key
RJMUSRYZPJIFPJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Niclosamide is a teniacide in the anthelmintic family. It is effective against cestodes that infect humans. Niclosamide is used to study the Wnt/Frizzled-1 signaling pathway. It is used to inhibit transcription and DNA binding of the NF-?B pathway and it increases ROS levels to induce apoptosis in acute myelogenous leukemia (AML) cells.
Biochem/physiol Actions
Niclosamide uncouples oxidative phosphorylation in the tapeworm and inhibits mitochondrial oxidative phosphorylation of parasitic helminths. It blocks tumor necrosis factor-induced IκBα phosphorylation, translocation of p65, and expression of NF-κΒ– regulated genes in AML cells.
Other Notes
50g,250g
Keep container tightly closed in a dry and well-ventilated place.Keep in a dry place.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yanli Jin et al.
Cancer research, 70(6), 2516-2527 (2010-03-11)
NF-kappaB may be a potential therapeutic target for acute myelogenous leukemia (AML) because NF-kappaB activation is found in primitive human AML blast cells. In this report, we initially discovered that the potent antineoplastic effect of niclosamide, a Food and Drug
Mechanism of action of reagents that uncouple oxidative phosphorylation.
E C Weinbach et al.
Nature, 221(5185), 1016-1018 (1969-03-15)
Min Li et al.
The Journal of biological chemistry, 288(50), 35769-35780 (2013-11-01)
Autophagy can be activated via MTORC1 down-regulation by amino acid deprivation and by certain chemicals such as rapamycin, torin, and niclosamide. Lysosome is the degrading machine for autophagy but has also been linked to MTORC1 activation through the Rag/RRAG GTPase
Jones Gyamfi et al.
Scientific reports, 9(1), 11336-11336 (2019-08-07)
The microenvironment of breast cancer comprises predominantly of adipocytes. Adipocytes drive cancer progression through the secretion adipocytokines. Adipocytes induce epithelial mesenchymal transition of breast cancer cells through paracrine IL-6/Stat3 signalling. Treatment approaches that can target adipocytes in the microenvironment and
Yi-Te Yo et al.
Molecular cancer therapeutics, 11(8), 1703-1712 (2012-05-12)
A recent hypothesis for cancer chemoresistance posits that cytotoxic survival of a subpopulation of tumor progenitors drives the propagation of recurrent disease, underscoring the need for new therapeutics that target such primitive cells. To discover such novel compounds active against
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service