L7002
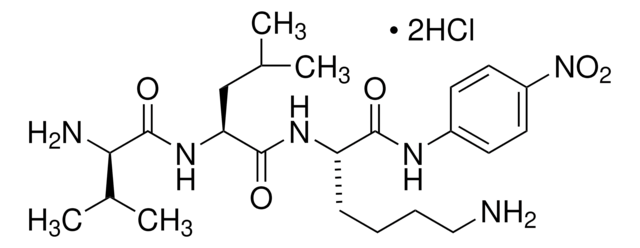
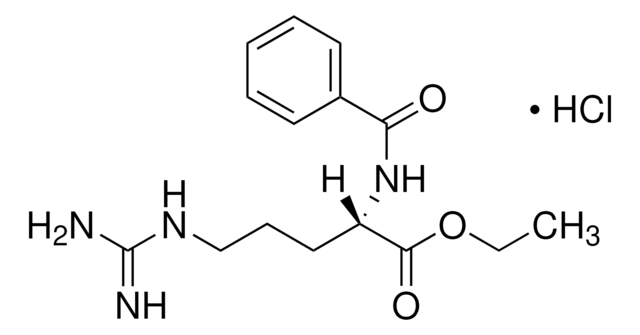
L-Lysine p-nitroanilide dihydrobromide
≥98% (TLC), suitable for ligand binding assays
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H18N4O3 · 2HBr
CAS Number:
Molecular Weight:
428.12
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
L-Lysine p-nitroanilide dihydrobromide,
Assay
≥98% (TLC)
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
white to off-white
storage temp.
2-8°C
SMILES string
Br.NCCCC[C@H](N)C(=O)Nc1ccc(cc1)N(=O)=O
InChI
1S/C12H18N4O3.BrH/c13-8-2-1-3-11(14)12(17)15-9-4-6-10(7-5-9)16(18)19;/h4-7,11H,1-3,8,13-14H2,(H,15,17);1H/t11-;/m0./s1
InChI key
FJNCJWJSSUJZMS-MERQFXBCSA-N
Application
L-Lysine p-nitroanilide may be used as a substrate to study the specificity and kinetics of lysine aminopeptidase(s) and various proteinases.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bernardo Ramírez-Zavala et al.
FEMS microbiology letters, 235(2), 369-375 (2004-06-09)
A lysine aminopeptidase was purified from the yeast Kluyveromyces marxianus. This enzyme was purified 100-fold from a soluble extract obtained at 100,000g. The purification procedure consisted in fractionated precipitation with ammonium sulfate and five chromatography steps. The native enzyme had
Akifumi Kawamura et al.
Biomacromolecules, 6(2), 627-631 (2005-03-15)
The amidase activity of bovine pancreas trypsin in water-soluble complexes with poly(ethylene glycol)-block-poly(alpha,beta-aspartic acid) (PEG-PAA) was evaluated by a colorimetric assay using L-lysine p-nitroanilide as a substrate. The enzymatic reaction of trypsin was accelerated through the complexation with PEG-PAA. By
Natasa Bozić et al.
Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology, 134(2), 231-241 (2003-02-06)
Exopeptidases of Morimus funereus larvae were partially purified and characterized. Specific leucyl aminopeptidase (LAP) activity was increased eight-fold by gel filtration of the crude midgut extract. The partially purified LAP had a molecular mass greater than 100 kDa with pH
Priscilla L Phillips et al.
PloS one, 13(11), e0207295-e0207295 (2018-11-13)
The oral obligate anaerobe Porphyromonas gingivalis possesses a small conserved transcript PG_RS02100 of unknown function we previously identified using small RNA-seq analysis as expressed during logarithmic growth. In this study, we sought to determine if PG_RS02100 plays a role in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service