F8512
Anti-Fibrinogen antibody produced in goat
whole antiserum
Synonym(s):
Anti-Fib2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
goat
Quality Level
conjugate
unconjugated
antibody form
whole antiserum
antibody product type
primary antibodies
clone
polyclonal
contains
15 mM sodium azide
species reactivity
human
technique(s)
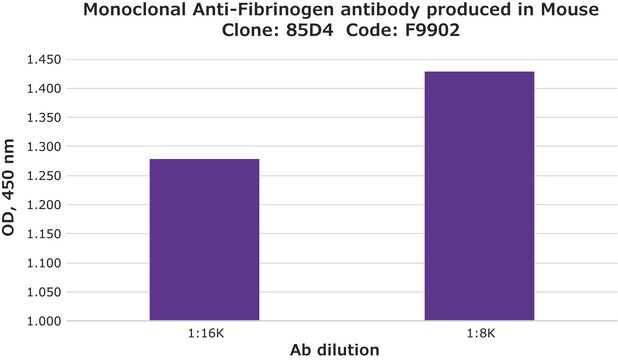
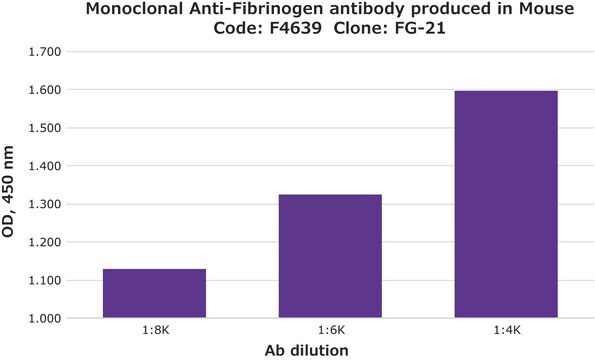
indirect ELISA: 1:10,000
quantitative precipitin assay: 2.0 mg/mL
UniProt accession no.
storage temp.
−20°C
target post-translational modification
unmodified
General description
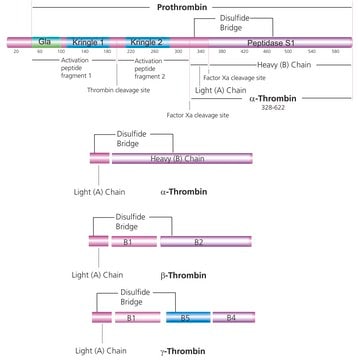
Fibrinogen is a thrombin-coagulable soluble plasma 340 kDa glycoprotein, composed of paired sets of three subunits i.e. α, β, γ. It plays a crucial role in protecting the vascular network against the loss of blood after tissue injury. Among three subunits, β and γ subunits contain one N-glycosylation site, which is occupied by a biantennary N-glycan. It contains three pairs of disulfide-bonded chains called α, β and γ which further folded into four structural domains: the D, E, connector, and the COOH-terminal region of the Aα chain.
The fibrinogen gene cluster consists of fibrinogen α, β and γ chains. It is localized on human chromosome locus 4q31.3−4q32.1.
Specificity
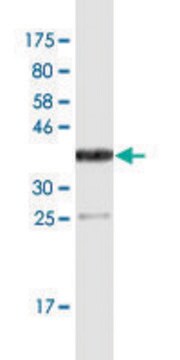
The antiserum has been determined to be immunospecific for fibrinogen by immunoelectrophoresis versus human plasma and fibrinogen.
Immunogen
human fibrinogen
Application
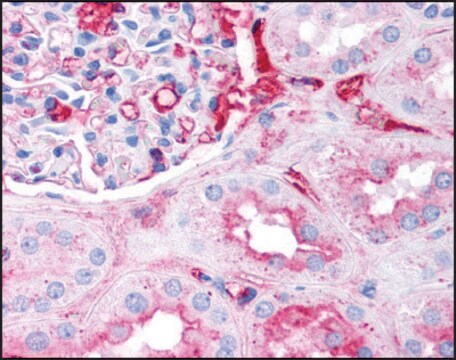
Anti-Fibrinogen antibody is suitable for immunostaining in fibrin deposition analysis of mouse livers and capturing antibodies in the sandwich ELISA. It is also suitable for indirect ELISA at a dilution of 1:10,000 and quantitative precipitin assay at 2.0mg/mL concentration.
Anti-Fibrinogen antibody produced in goat has been used in:
- western blotting detection in human colon adenocarcinoma cell line
- detecting fibrinogen in plasma
- immunoassay of human platelet free plasma (PFP)
Biochem/physiol Actions
Plasmin attacks the Aα chain COOH domain to produce the heterogeneous fragment X. Multiple round of degradation ended with terminal digestion products−fragments D and E which represent the major globular domains in fibrinogen. Mutations in this gene lead to several disorders, including hypofibrinogenemia and afibrinogenemia.
Preparation Note
treated to remove lipoproteins
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Congenital afibrinogenaemia caused by uniparental isodisomy of chromosome 4 containing a novel 15-kb deletion involving fibrinogen Aalpha-chain gene.
Spena S, et al.
European Journal of Human Genetics, 12(11), 891-891 (2004)
Integrin alphavbeta6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn2+.
Fouchier F, et al.
European Journal of Cell Biology, 86(3), 143-160 (2007)
A quantitative immunoassay for lung cancer biomarker CIZ1b in patient plasma.
Coverley D, et al.
Clinical Biochemistry, 50(6), 336-343 (2017)
Platelet adhesion and plasma protein adsorption control of collagen surfaces by He+ ion implantation.
Kurotobi K, et al.
Nucl. Instrum. Methods Phys. Res. Sect. B, 206(6), 532-537 (2003)
N E Kirschbaum et al.
The Journal of biological chemistry, 265(23), 13669-13676 (1990-08-15)
The COOH-terminal portion of the A alpha chain of human fibrinogen is highly susceptible to proteolytic degradation. This property has prevented isolation of the COOH-terminal domain of fibrinogen for the direct investigation of its functional characteristics. Human fibrinogen was degraded
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service