C3974
Ciglitizone
≥98% (HPLC)
Synonym(s):
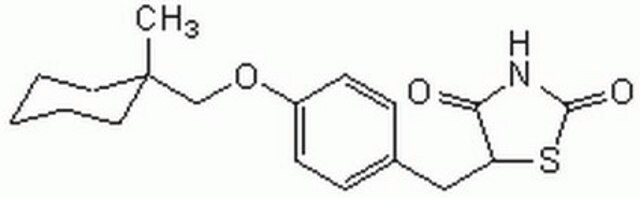
(±)-5-[4-(1-Methylcyclohexylmethoxy)benzyl]thiazolidine-2,4-dione, Ciglitazone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H23NO3S
CAS Number:
Molecular Weight:
333.45
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
originator
Takeda
SMILES string
CC1(CCCCC1)COc2ccc(CC3SC(=O)NC3=O)cc2
InChI
1S/C18H23NO3S/c1-18(9-3-2-4-10-18)12-22-14-7-5-13(6-8-14)11-15-16(20)19-17(21)23-15/h5-8,15H,2-4,9-12H2,1H3,(H,19,20,21)
InChI key
YZFWTZACSRHJQD-UHFFFAOYSA-N
Gene Information
human ... PPARG(5468)
mouse ... Pparg(19016)
Application
Ciglitizone has been used as a proliferator-activated receptor γ (PPARγ) agonist:
It also may be used to study its effects on cell cycle and apoptosis in monocytic cells.
- to study its effects on cell proliferation in human melanocytes
- to study its effects on pigmentation and migration of human melanocytes
It also may be used to study its effects on cell cycle and apoptosis in monocytic cells.
Biochem/physiol Actions
Ciglitizone belongs to the class of thiazolidinediones and is a peroxisome proliferator-activated receptor γ (PPARγ) agonist. It exhibits anti-diabetic activity. Ciglitizone has the potential to treat tumor necrosis factor α (TNFα)-related apoptosis-inducing ligand (TRAIL)-refractory high-grade urothelial cancers.
Features and Benefits
This compound is a featured product for Gene Regulation research. Click here to discover more featured Gene Regulation products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound was developed by Takeda. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eun Jong Han et al.
International journal of radiation oncology, biology, physics, 85(5), e239-e248 (2013-01-22)
To investigate possible radiosensitizing activities of the well-known peroxisome proliferator-activated receptor (PPAR)γ ligand ciglitazone and novel PPARγ ligands CAY10415 and CAY10506 in non-small cell lung cancer (NSCLC) cells. Radiosensitivity was assessed using a clonogenic cell survival assay. To investigate the
S Zulkafli Nor Effa et al.
Biomolecules, 8(4) (2018-11-08)
Immunomodulation, as a means of immunotherapy, has been studied in major research and clinical laboratories for many years. T-Regulatory (Treg) cell therapy is one of the modulators used in immunotherapy approaches. Similarly, nuclear receptor peroxisome proliferator activated receptor gamma (PPARγ)
The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones.
T M Willson et al.
Journal of medicinal chemistry, 39(3), 665-668 (1996-02-02)
H Y Kang et al.
The British journal of dermatology, 150(3), 462-468 (2004-03-20)
Peroxisome proliferator-activated receptors (PPARs) belong to the superfamily of nuclear receptors that heterodimerize with the retinoic X receptor. Agonists of PPAR have been known to play an important role in cellular responses including proliferation and differentiation. The expression and function
[[omega-(Heterocyclylamino)alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents.
B C Cantello et al.
Journal of medicinal chemistry, 37(23), 3977-3985 (1994-11-11)
A series of [(ureidoethoxy)benzyl]-2,4-thiazolidinediones and [[(heterocyclylamino)alkoxy]-benzyl]-2,4-thiazolidinediones was synthesized from the corresponding aldehydes. Compounds from the urea series, exemplified by 16, showed antihyperglycemic potency comparable with known agents of the type such as pioglitazone and troglitazone (CS-045). The benzoxazole 49, a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service