49180
Glucose Oxidase from Aspergillus niger
lyophilized, powder, ~200 U/mg
Synonym(s):
β-D-Glucose:oxygen 1-oxidoreductase, G.Od., GOx
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Aspergillus niger
form
powder
quality
lyophilized
specific activity
~200 U/mg
mol wt
Mr ~186000
foreign activity
catalase ≤1%
shipped in
dry ice
storage temp.
−20°C
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1
InChI key
WQZGKKKJIJFFOK-VFUOTHLCSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Molecular Weight: 160 kDa (gel filtration)
pI: 4.2
Extinction coefficient: E1% = 16.7 (280 nm)
Glucose oxidase from Aspergillus niger is a dimer consisting of 2 equal subunits with a molecular mass of 80 kDa each. Each subunit contains one flavin adenine dinulceotide moiety and one iron. The enzyme is a glycoprotein containing ~16% neutral sugar and 2% amino sugars. The enzyme also contains 3 cysteine residues and 8 potential sites for N-linked glycosylation.
Glucose oxidase is capable of oxidizing D-aldohexoses, monodeoxy-D-glucoses, and methyl-D-glucoses at varying rates.
The pH optimum for glucose oxidase is 5.5, while it has a broad activity range of pH 4-7. Glucose oxidase is specific for β-D-glucose with a KM of 33-110 mM.
Glucose oxidase does not require any activators, but it is inhibited by Ag+, Hg2+, Cu2+, phenylmercuric acetate, and p-chloromercuribenzoate. It is not inhibited by the nonmetallic SH reagents: N-ethylmaleimide, iodoacetate, and iodoacetamide.
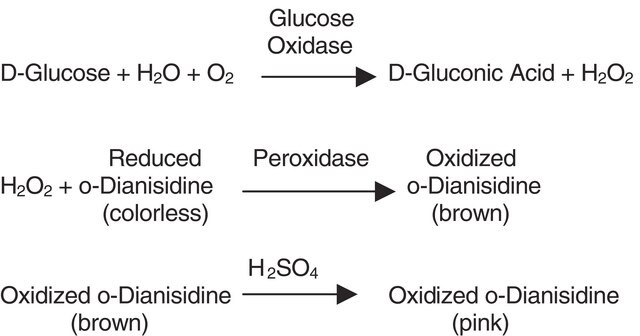
Glucose oxidase can be utilized in the enzymatic determination of D-glucose in solution. As glucose oxidase oxidizes β-D-glucose to D-gluconolactate and hydrogen peroxide, horseradish peroxidase is often used as the coupling enzyme for glucose determination. Although glucose oxidase is specific for β-D-glucose, solutions of D-glucose can be quantified as α-D-glucose will mutorotate to β-D-glucose as the β-D-glucose is consumed by the enzymatic reaction.
pI: 4.2
Extinction coefficient: E1% = 16.7 (280 nm)
Glucose oxidase from Aspergillus niger is a dimer consisting of 2 equal subunits with a molecular mass of 80 kDa each. Each subunit contains one flavin adenine dinulceotide moiety and one iron. The enzyme is a glycoprotein containing ~16% neutral sugar and 2% amino sugars. The enzyme also contains 3 cysteine residues and 8 potential sites for N-linked glycosylation.
Glucose oxidase is capable of oxidizing D-aldohexoses, monodeoxy-D-glucoses, and methyl-D-glucoses at varying rates.
The pH optimum for glucose oxidase is 5.5, while it has a broad activity range of pH 4-7. Glucose oxidase is specific for β-D-glucose with a KM of 33-110 mM.
Glucose oxidase does not require any activators, but it is inhibited by Ag+, Hg2+, Cu2+, phenylmercuric acetate, and p-chloromercuribenzoate. It is not inhibited by the nonmetallic SH reagents: N-ethylmaleimide, iodoacetate, and iodoacetamide.
Glucose oxidase can be utilized in the enzymatic determination of D-glucose in solution. As glucose oxidase oxidizes β-D-glucose to D-gluconolactate and hydrogen peroxide, horseradish peroxidase is often used as the coupling enzyme for glucose determination. Although glucose oxidase is specific for β-D-glucose, solutions of D-glucose can be quantified as α-D-glucose will mutorotate to β-D-glucose as the β-D-glucose is consumed by the enzymatic reaction.
Application
Glucose oxidase is widely used in the food and pharmaceutical industries as well as a major component of glucose biosensors.
Biochem/physiol Actions
Glucose oxidase catalyses the oxidation of β-d-glucose to d-glucono-β-lactone and hydrogen peroxide, with molecular oxygen as an electron acceptor.
Unit Definition
1 U corresponds to the amount of enzyme which oxidizes 1 μmol glucose per minute at pH 7.0 and 25 °C
One unit will oxidize 1.0 μmole of β-D-glucose to D-gluconolactone and H2O2 per min at pH 5.1 at 35 °C, equivalent to an O2 uptake of 22.4 μl per min. If the reaction mixture is saturated with oxygen, the activity may increase by up to 100%.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alex J Wadley et al.
Methods in molecular biology (Clifton, N.J.), 1990, 53-70 (2019-05-31)
Increased production of reactive oxygen species (ROS) and deficiencies in cellular antioxidant defenses are the principal causes of cellular oxidative stress. ROS can react with a variety intracellular molecules, including redox active cysteine thiols (-SH) within proteins. Cysteine thiols can
Zhichao Fan et al.
Cell reports, 26(1), 119-130 (2019-01-04)
Leukocyte adhesion requires β2-integrin activation. Resting integrins exist in a bent-closed conformation-i.e., not extended (E-) and not high affinity (H-)-unable to bind ligand. Fully activated E+H+ integrin binds intercellular adhesion molecules (ICAMs) expressed on the opposing cell in trans. E-H-
Geetika Aggarwal et al.
The Journal of biological chemistry, 297(3), 101019-101019 (2021-08-01)
Reduced activity of paraoxonase 1 (PON1), a high-density lipoprotein (HDL)-associated enzyme, has been implicated in the development of atherosclerosis. Post-translational modifications of PON1 may represent important mechanisms leading to reduced PON1 activity. Under atherosclerotic conditions, myeloperoxidase (MPO) is known to
Man Sup Kwak et al.
Redox biology, 24, 101203-101203 (2019-04-27)
The nuclear protein HMGB1 (high mobility group box 1) is secreted by monocytes-macrophages in response to inflammatory stimuli and serves as a danger-associated molecular pattern. Acetylation and phosphorylation of HMGB1 are implicated in the regulation of its nucleocytoplasmic translocation for
Zhengpeng Yang et al.
Biosensors & bioelectronics, 51, 268-273 (2013-08-27)
Core-shell Fe3O4-enzyme-polypyrrole (Ppy) nanoparticles with excellent magnetism and conductivity were successfully prepared via the surface modification and enzyme self-encapsulation within Ppy. A novel potentiometric glucose biosensor has been constructed by effectively attaching the proposed Fe3O4-enzyme-Ppy nanoparticles to the surface of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service