About This Item
Recommended Products
grade
ACS reagent
Quality Level
vapor density
1.6 (vs air)
vapor pressure
44.8 mmHg ( 20 °C)
Assay
≥96%
form
liquid
autoignition temp.
1004 °F
expl. lim.
57 %
refractive index
n20/D 1.370 (lit.)
pH
2.2 (20 °C, 2.2 g/L)
bp
100-101 °C (lit.)
mp
8.2-8.4 °C (lit.)
solubility
water: miscible
density
1.22 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤0.001%
sulfate (SO42-): ≤0.003%
sulfite (SO32-): passes test
cation traces
Fe: ≤0.001%
NH4+: ≤0.005%
heavy metals (as Pb): ≤0.001%
application(s)
sample preparation
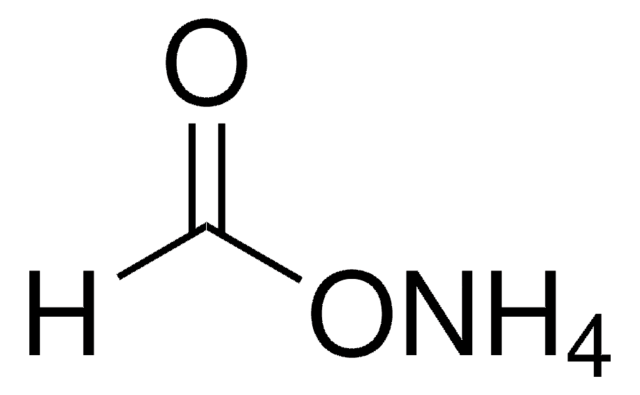
SMILES string
OC=O
InChI
1S/CH2O2/c2-1-3/h1H,(H,2,3)
InChI key
BDAGIHXWWSANSR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Co-Nx-catalyzed one-pot reductive amination of carbonyl compounds with nitro compounds to synthesize secondary amines.
- Photoreduction of polycyclic and heterocyclic nitro arenes to corresponding amines.
- Pd-catalyzed chemoselective hydrogenation of olefins to saturated hydrocarbons.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
121.1 °F - closed cup
Flash Point(C)
49.5 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service