70650
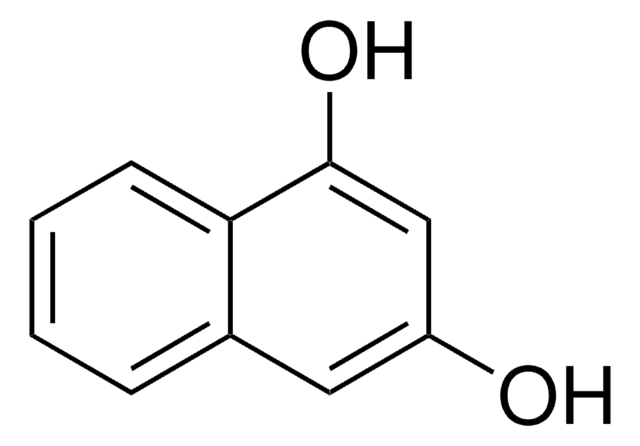
1,3-Dihydroxynaphthalene
for spectrophotometric det. of glucuronic acid according to Tollens, ≥97.0%
Synonym(s):
1,3-Naphthalenediol, Naphthoresorcinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C10H6(OH)2
CAS Number:
Molecular Weight:
160.17
Beilstein:
2044002
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥97.0% (HPLC)
≥97.0%
form
crystals
quality
for spectrophotometric det. of glucuronic acid according to Tollens
technique(s)
UV/Vis spectroscopy: suitable
mp
122-125 °C
123-125 °C (lit.)
SMILES string
Oc1cc(O)c2ccccc2c1
InChI
1S/C10H8O2/c11-8-5-7-3-1-2-4-9(7)10(12)6-8/h1-6,11-12H
InChI key
XOOMNEFVDUTJPP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,3-Dihydroxynaphthalene may be used as a background electrolyte (BGE) for the determination of carbohydrates by high-performance capillary electrophoresis (HPCE) method with indirect absorbance detection.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Determination of carbohydrates by high-performance capillary electrophoresis with indirect absorbance detection.
Lee YH and Lin TI
Journal of Chromatography. B, Biomedical Sciences and Applications, 681(1), 87-97 (1996)
Y Sayato et al.
Mutation research, 189(3), 217-222 (1987-11-01)
The mutagenicity of products formed by ozonation of naphthoresorcinol in aqueous solution was assayed with Salmonella typhimurium strains TA97, TA98, TA100, TA102 and TA104 in the presence and absence of S9 mix from phenobarbital- and 5,6-benzoflavone-induced rat liver. Ozonated naphthoresorcinol
Wilson Terán et al.
The Journal of biological chemistry, 281(11), 7102-7109 (2006-01-13)
The RND family transporter TtgABC and its cognate repressor TtgR from Pseudomonas putida DOT-T1E were both shown to possess multidrug recognition properties. Structurally unrelated molecules such as chloramphenicol, butyl paraben, 1,3-dihydroxynaphthalene, and several flavonoids are substrates of TtgABC and activate
Determination of glycolaldehyde in the presence of hydroxypyruvate and tartronate semialdehyde.
F E de Windt et al.
Analytical biochemistry, 106(2), 342-343 (1980-08-01)
Amy L Kieran et al.
Chemical communications (Cambridge, England), (14)(14), 1842-1844 (2005-03-30)
New macrocycles incorporating a porphyrin and a [small pi] electron-rich aromatic were prepared from a dynamic disulfide library. The outcome could be influenced by use of templates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service