31581
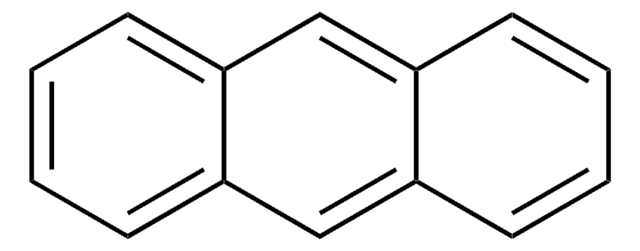
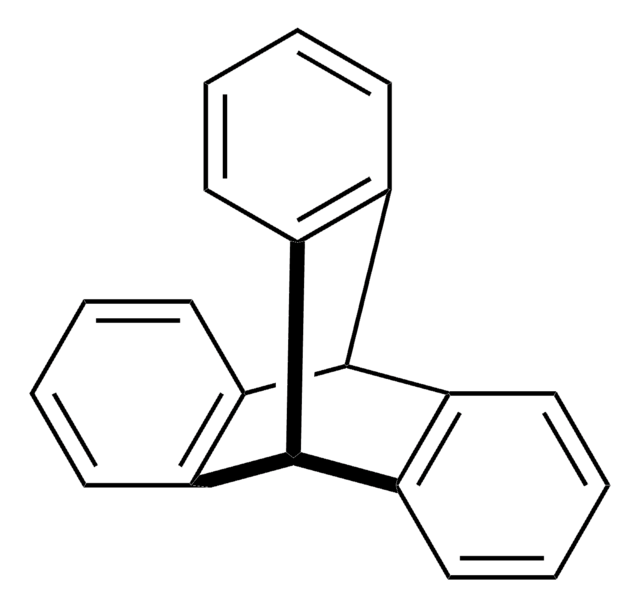
Anthracene
analytical standard
Synonym(s):
Anthraxcene, Paranaphthalene
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor density
6.15 (vs air)
vapor pressure
1 mmHg ( 145 °C)
autoignition temp.
1004 °F
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
340 °C (lit.)
mp
210-215 °C (lit.)
solubility
alcohols: soluble
benzene: soluble
chloroform: soluble
hydronaphthalenes: soluble
supercritical carbon dioxide: soluble
application(s)
environmental
format
neat
SMILES string
c1ccc2cc3ccccc3cc2c1
InChI
1S/C14H10/c1-2-6-12-10-14-8-4-3-7-13(14)9-11(12)5-1/h1-10H
InChI key
MWPLVEDNUUSJAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Recommended products
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
249.8 °F - closed cup
Flash Point(C)
121.0 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH SVHC Candidate List
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
US EPA Method 8270 (PAH only): GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
HPLC Analysis of PAHs on SUPELCOSIL™ LC-PAH
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service