654380
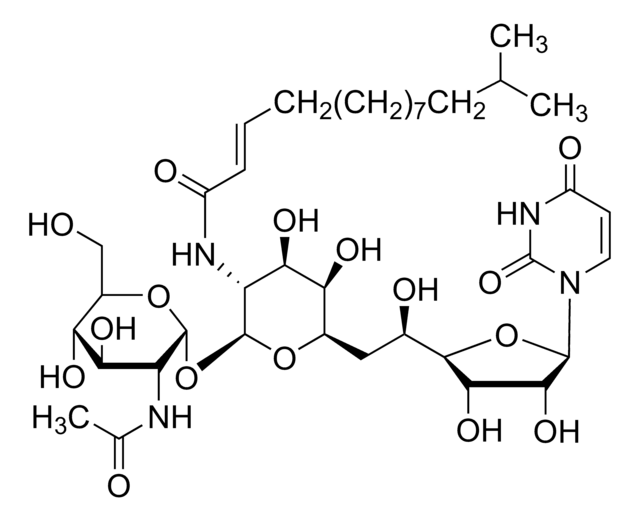
Tunicamycin
from Strepomyces lysosuperficus, ≥98% (A+B+C+D, HPLC), powder, N-acetylglucosamine transferase, Calbiochem
Synonym(s):
Tunicamycin, Streptomyces lysosuperficus
About This Item
Recommended Products
Product Name
Tunicamycin, Streptomyces lysosuperficus,
description
Tunicamycin, Streptomyces lysosuperficus
Quality Level
Assay
≥98% (A+B+C+D, HPLC)
form
powder
manufacturer/tradename
Calbiochem®
solubility
DMSO: 10 mg/mL
DMF: soluble
pyridine: soluble
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C30H46N4O16/c1-11(2)5-4-6-16(38)32-19-23(43)20(40)14(47-29(19)50-28-18(31-12(3)36)22(42)21(41)15(10-35)48-28)9-13(37)26-24(44)25(45)27(49-26)34-8-7-17(39)33-30(34)46/h4,6-8,11,13-15,18-29,35,37,40-45H,5,9-10H2,1-3H3,(H,31,36)(H,32,38)(H,33,39,46)/b6-4+/t13?,14-,15-,18-,19-,20+,21-,22-,23-,24+,25-,26-,27-,28-,29-/m1/s1
InChI key
ZHSGGJXRNHWHRS-PEALBESXSA-N
General description
Biochem/physiol Actions
N-linked glycosylation
thrombin-induced Ca2+ mobilization in cells
Warning
Preparation Note
Reconstitution
Other Notes
Price, B.D., et al. 1992. J. Cell Physiol.152, 545.
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![PERK Inhibitor I, GSK2606414 GSK2606414 is a cell-permeable, highly potent inhibitor of EIF2AK3/PERK (IC₅₀ = 0.4 nM; [ATP] = 5 µM). Targets PERK in its inactive DFG conformation at the ATP-binding region.](/deepweb/assets/sigmaaldrich/product/structures/180/559/efa716dc-d5fe-4339-a6f0-0103084fc04a/640/efa716dc-d5fe-4339-a6f0-0103084fc04a.png)