All Photos(3)
About This Item
Empirical Formula (Hill Notation):
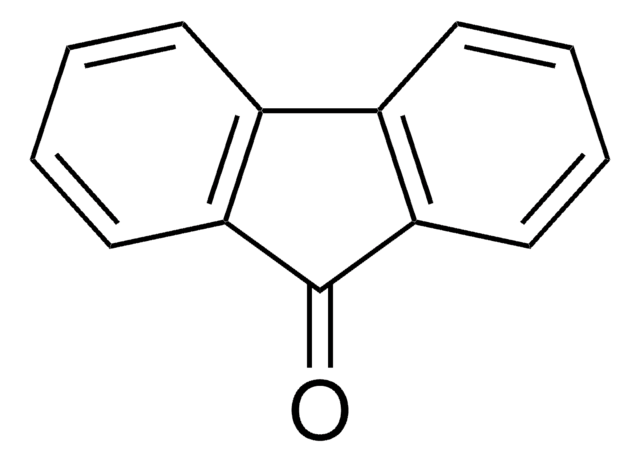
C13H10O
CAS Number:
Molecular Weight:
182.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
153-154 °C (lit.)
SMILES string
OC1c2ccccc2-c3ccccc13
InChI
1S/C13H10O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8,13-14H
InChI key
AFMVESZOYKHDBJ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Bastiaens et al.
Research in microbiology, 152(10), 849-859 (2002-01-05)
The promoter probe mini-Tn5-luxAB-tet was used to create a luxAB transcriptional fusion responding to fluorene in the fluorene utilising bacterium Sphingomonas sp. LB126. The mutant strain, named L-132, was impaired in fluorene utilisation and strongly emitted light upon addition of
Derek Dunn et al.
Bioorganic & medicinal chemistry letters, 22(11), 3751-3753 (2012-05-02)
In searching for a next generation molecule to the novel wake promoting agent modafinil (compound 1), a series of fluorene-derived wakefulness enhancing agents were developed and evaluated in rat. Extensive pharmacokinetic studies of a potent member of the series (compound
C Chen et al.
Drug metabolism and disposition: the biological fate of chemicals, 12(4), 421-426 (1984-07-01)
Fluoren-9-ol and fluoren-9-one were used as model substrates to study microsomal metabolism of alcohols and carbonyl compounds. It was found that there was an oxidoreductase(s) present in the microsomal preparation that catalyzed interconversion of this alcohol and ketone using pyridine
Microbial reduction of 9-fluorenone to 9-hydroxyfluorene in carbon-filtered water: a confounding factor in an aquatic bioassay of 9-fluorenone with larvae of the midge, Chironomus tentans (Fabr.).
P W O'Keefe et al.
Bulletin of environmental contamination and toxicology, 75(6), 1060-1066 (2006-01-13)
Biban Gill et al.
Analytical and bioanalytical chemistry, 411(7), 1397-1407 (2019-01-27)
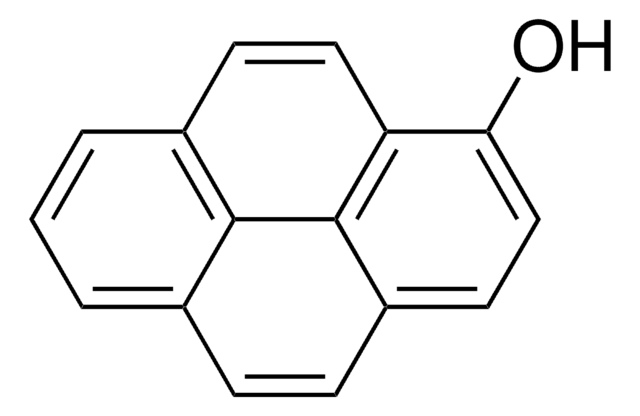
Urinary 1-hydroxypyrene (OH-Pyr) is widely used for biomonitoring human exposures to polycyclic aromatic hydrocarbons (PAHs) from air pollution and tobacco smoke. However, there have been few rigorous validation studies reported to ensure reliable OH-Pyr determination for occupational health and risk
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service