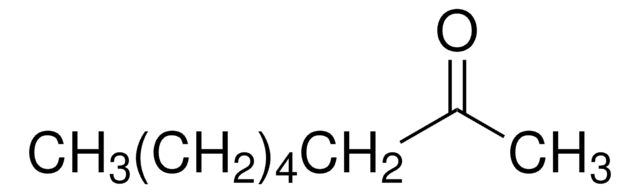E29109
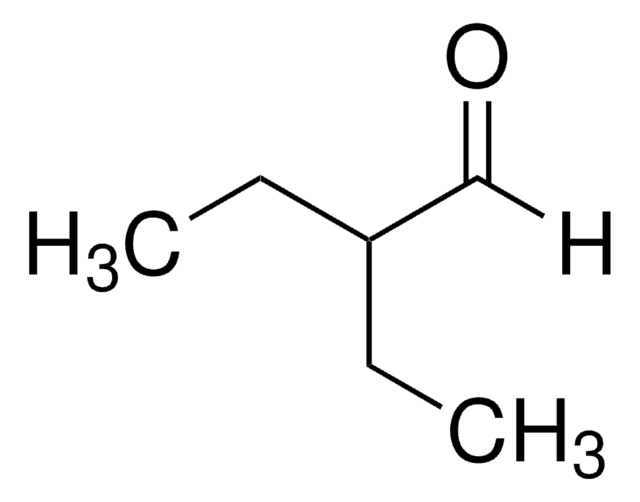
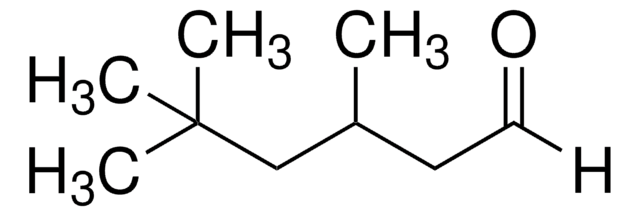
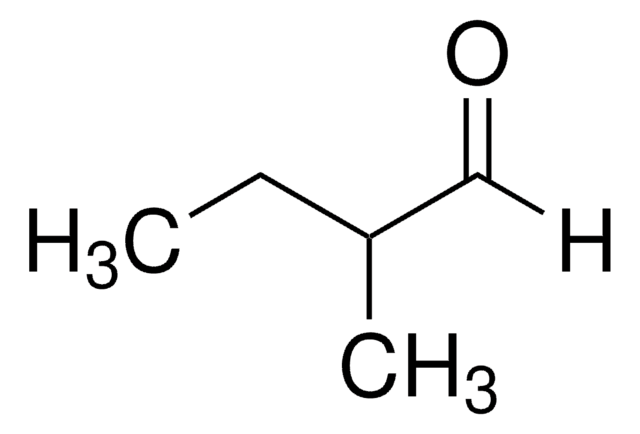
2-Ethylhexanal
96%
Synonym(s):
2-Ethylcapronaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
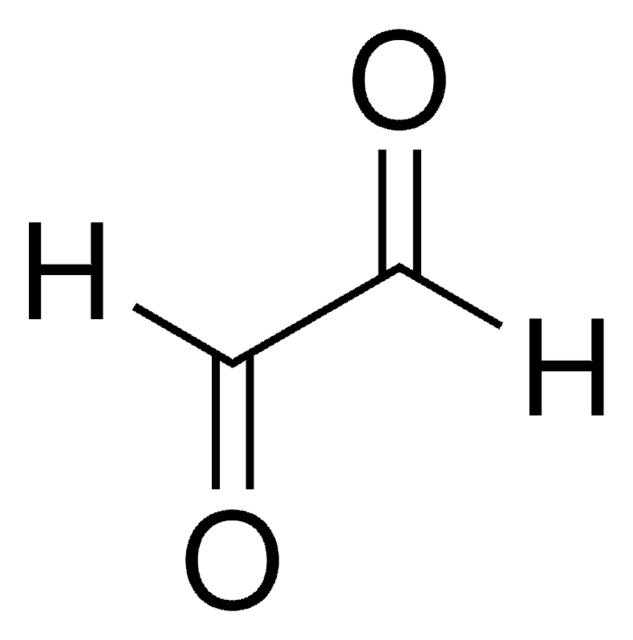
CH3(CH2)3CH(C2H5)CHO
CAS Number:
Molecular Weight:
128.21
Beilstein:
1700556
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
impurities
<4% 2-ethylhexanoic acid
refractive index
n20/D 1.415 (lit.)
bp
55 °C/13.5 mmHg (lit.)
density
0.822 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)C(CC)CCCC
InChI
1S/C8H16O/c1-3-5-6-8(4-2)7-9/h7-8H,3-6H2,1-2H3
InChI key
LGYNIFWIKSEESD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Ethylhexanal is an aliphatic aldehyde used as an intermediate to produce various useful products such as perfumes, disinfectants, paints, warning agents, insecticides, and leak detectors.
Application
2-Ethylhexanal can be used as:
- A reductant in the Mukaiyama epoxidation of cis-cyclooctene.
- A reactant in the Horner-Wadsworth-Emmons reaction to synthesize cis-α,β-unsaturated amides.
- A starting material to synthesize 2-ethylhexanoic acid using manganese(II) acetate as a catalyst.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Repr. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Factors affecting the selectivity of air oxidation of 2-ethyhexanal, an α-branched aliphatic aldehyde
Lehtinen C and Brunow G
Organic Process Research & Development, 4, 544-549 (2000)
David M Hodgson et al.
The Journal of organic chemistry, 78(4), 1508-1518 (2013-01-30)
The synthesis and alkylation of chiral, nonracemic tropane- and homotropane-derived enamines is examined as an approach to enantioenriched α-alkylated aldehydes. The two bicyclic N auxiliaries, which differ by a single methylene group, give opposite senses of asymmetric induction on alkylation
The effect of feeding di-(2-ethylhexyl) phthalate and related compounds on lipids in the laying hen.
D L Wood et al.
Poultry science, 63(3), 469-477 (1984-03-01)
Di-(2-ethylhexyl) phthalate (DEHP) and three structurally related side-chain analogs were fed to laying hens to determine relationships of structure to effects on lipid metabolism. Hubbard broiler breeder hens were fed either a standard laying mash control diet or the control
G Casperson et al.
Journal of basic microbiology, 26(5), 259-269 (1986-01-01)
Analogs of the natural substance (E)-hex-2-en-1-al which occurs naturally in green plants and is known to have microbiocidal properties were synthesized and studied with respect to their antifungal properties. Saturated and unsaturated aldehydes, alcohols, carbon acids and enolacetate were tested
Heather L Voegtle et al.
Journal of chemical ecology, 34(2), 215-219 (2008-01-24)
E-2-ethyl-2-hexen-1-ol (1), mellein (4), and 4-hydroxymellein (5) were identified as the major volatile compounds in the head and/or thorax of Camponotus quadrisectus. Neither 1 nor 5 have been previously detected in insects. Also identified were small amounts of m-cresol (2)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service