C92006
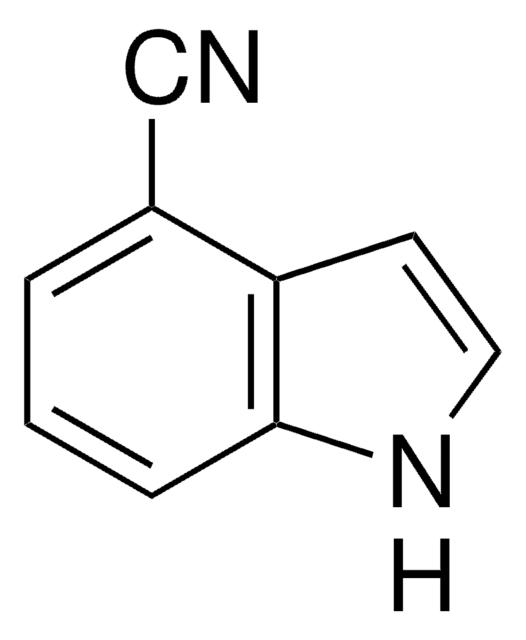
Indole-5-carbonitrile
99%
Synonym(s):
5-Cyanoindole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6N2
CAS Number:
Molecular Weight:
142.16
Beilstein:
116738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
106-108 °C (lit.)
SMILES string
N#Cc1ccc2[nH]ccc2c1
InChI
1S/C9H6N2/c10-6-7-1-2-9-8(5-7)3-4-11-9/h1-5,11H
InChI key
YHYLDEVWYOFIJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Reactant for parallel synthesis of dihydroisoquinolines via silver and L-proline co-catalyzed three-component coupling reaction
- Reactant for chemoselective and regioselective preparation of benzoyl indoles
- Reactant for preparation of novel PPARα/γ dual agonists for potential treatment of metabolic syndrome and IDDM
- Reactant for preparation of 4,5-dihydrocyclopenta[c]quinolines by palladium-catalyzed ring-expansion reaction alkynes, using O2 as the oxidant
- Reactant for preparation of vinylindoles by hydroarylation of alkynes using indium bromide catalyst
- Reactant for gold catalyzed direct alkynylation using a benziodoxolone based hypervalent iodine reagent
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Josefin Wilke et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 17(17), 2736-2743 (2016-06-02)
The estimate of the magnitude and the orientation of molecular electric dipole moments from the vector sum of bond or fragment dipole moments is a widely used approach in chemistry. However, the limitations of this intuitive model have rarely been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service