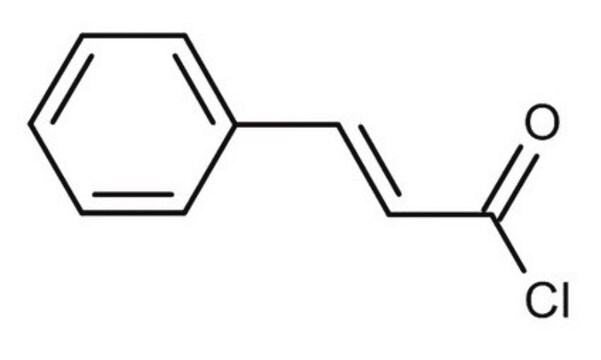
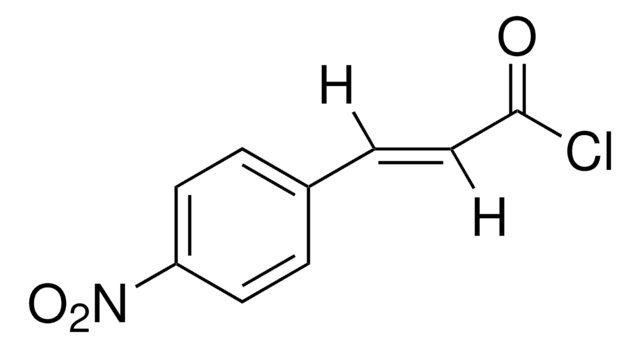
C81101
Cinnamoyl chloride

≥95.0%
Synonym(s):
trans-3-Phenylacryloyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
C6H5CH=CHCOCl
CAS Number:
Molecular Weight:
166.60
Beilstein:
606265
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
bp
256-258 °C (lit.)
mp
35-37 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(=O)\C=C\c1ccccc1
InChI
1S/C9H7ClO/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
WOGITNXCNOTRLK-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C J Eboka et al.
Journal of pharmaceutical sciences, 72(4), 366-369 (1983-04-01)
The kinetics of reaction of the acylating agents trans-cinnamic anhydride and trans-cinnamoyl chloride with the hydroxy compounds n-propyl alcohol and water in the presence of N-methylimidazole and 4-dimethylaminopyridine were studied spectrophotometrically in acetonitrile solution at 25 degrees. The acid chloride
María V Buchieri et al.
Bioorganic & medicinal chemistry letters, 23(3), 740-743 (2012-12-26)
A small series of C-cinnamoyl glycoside containing the phenol moiety was tested for the inhibition of the three Mycobacterium tuberculosis β-carbonic anhydrases (CAs, EC 4.2.1.1) with activities in the low micromolar range detected. The compounds were also tested for the
K A Connors et al.
Journal of pharmaceutical sciences, 72(4), 369-372 (1983-04-01)
The kinetics of reaction of trans-cinnamic anhydride or trans-cinnamoyl chloride with n-propyl alcohol, catalyzed by N-methylimidazole or 4-dimethylaminopyridine, were studied spectrophotometrically at 25 degrees in methyl ethyl ketone, ethylene dichloride, methylene chloride, and toluene. The acid chloride reacted in all
Yukiya Kitayama et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(52), 12870-12875 (2017-06-29)
In this study, a fabrication route towards functional capsule particles was successfully developed by means of a self-templating shell-selective cross-linking strategy that enables us to prepare shell-cross-linked hollow polymer particles directly from homogeneous spherical polymer particles. To prepare redox-responsive degradable
Shinya Yano et al.
Carbohydrate polymers, 184, 418-426 (2018-01-22)
Biocompatibility of cinnamoyl-modified carbohydrate materials is not well-known, while they are attracting attention as a photoreactive material. In order to investigate biocompatible properties of cinnamoyl-modified carbohydrate, hydroxypropyl cellulose (HPC) was reacted with cinnamoyl chloride to yield cinnamoyl-modified HPC (HPC-C) for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service