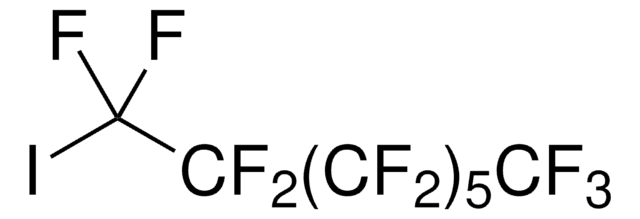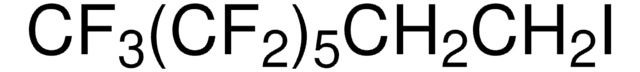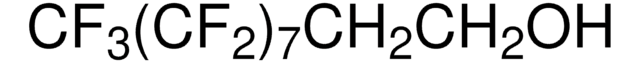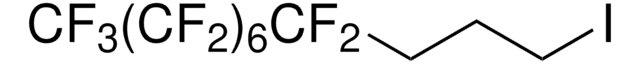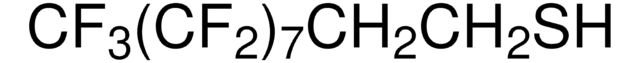91987
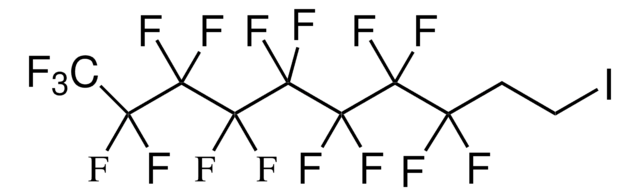
1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10-Henicosafluoro-12-iodododecane
≥95.0% (GC)
Synonym(s):
1-Iodo-1H,1H,2H,2H-perfluorododecane, 1H,1H,2H,2H-Perfluorododecyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3(CF2)9CH2CH2I
CAS Number:
Molecular Weight:
674.03
Beilstein:
1897869
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (GC)
form
powder
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)CCI
InChI
1S/C12H4F21I/c13-3(14,1-2-34)4(15,16)5(17,18)6(19,20)7(21,22)8(23,24)9(25,26)10(27,28)11(29,30)12(31,32)33/h1-2H2
InChI key
HVWXRMINOYZYCK-UHFFFAOYSA-N
Other Notes
Building block to introduce a fluorinated "pony tail" in compounds for fluorous phase «immobilization» by fluorous phase partition
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wilson et al.
The Journal of organic chemistry, 65(9), 2619-2623 (2000-05-16)
Fullerene (C60) Diels-Alder adducts with perfluoroalkylated 1,3-cyclopentadiene (1a,b) were synthesized and studied. The perfluoroalkylated cyclopentadiene was found to be less reactive toward C60 than cyclopentadiene itself, possibly because of the electron-withdrawing effect of the side chain. Because of the same
Armido Studer et al.
The Journal of organic chemistry, 62(9), 2917-2924 (1997-05-02)
A new protocol for multicomponent condensation reactions that uses fluorous (highly fluorinated) substrates is introduced. This method takes advantage of the ease of purification of fluorous compounds by liquid-liquid extractions between fluorous and organic solvents. The application of this method
R.P. Hughes, H.A. Trujillo
Organometallics, 15, 286-286 (1996)
Daniel Rauber et al.
Physical chemistry chemical physics : PCCP, 19(40), 27251-27258 (2017-10-11)
Ionic liquids (ILs) exhibit tunable behaviour and properties that are due to their supramolecular structure. We synthesized a series of alkylated and fluorinated phosphonium dicyanamide ILs to study the relation between molecular structure and assembly with a focus on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service