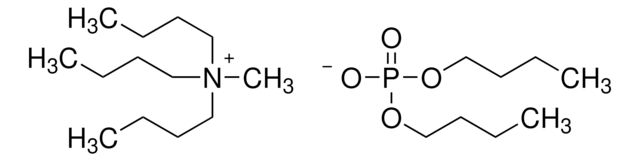
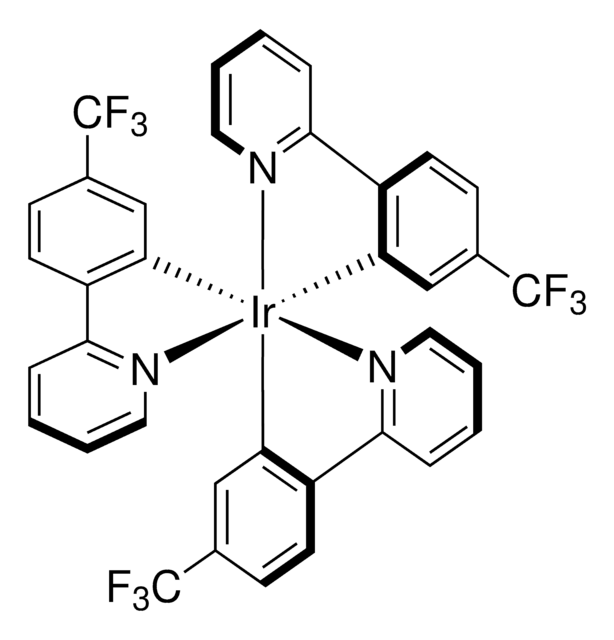
908568
[Ir(dFCF3ppy)2-(5,5′-dCF3bpy)]PF6
≥95%
Synonym(s):
[5,5′-Bis(trifluoromethyl)-2,2′-bipyridine-N1,N1′]bis[3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]Iridium(III) hexafluorophosphate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C36H16F22IrN4P
CAS Number:
Molecular Weight:
1145.69
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
mp
>300 °C
photocatalyst activation
460 nm
Related Categories
Application
[Ir(dFCF3ppy)2-(5,5′-dCF3bpy)]PF6 is a cyclometalated iridium(III) complex that can be used in visible-light mediated photocatalytic organic transformations including the alkylation of remote C-H bonds and alkene aminoarylation.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gilbert J Choi et al.
Nature, 539(7628), 268-271 (2016-11-04)
Despite advances in hydrogen atom transfer (HAT) catalysis, there are currently no molecular HAT catalysts that are capable of homolysing the strong nitrogen-hydrogen (N-H) bonds of N-alkyl amides. The motivation to develop amide homolysis protocols stems from the utility of
Timothy M Monos et al.
Science (New York, N.Y.), 361(6409), 1369-1373 (2018-09-29)
Alkene aminoarylation with a single, bifunctional reagent is a concise synthetic strategy. We report a catalytic protocol for the addition of arylsulfonylacetamides across electron-rich alkenes with complete anti-Markovnikov regioselectivity and excellent diastereoselectivity to provide 2,2-diarylethylamines. In this process, single-electron alkene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)
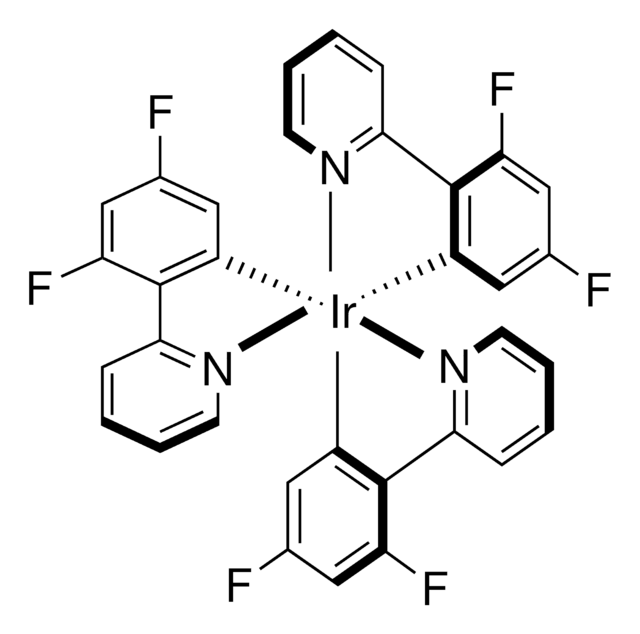
![[Ir(dFppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/258/715/c8fe85d5-be71-4ff1-849b-a20766636770/640/c8fe85d5-be71-4ff1-849b-a20766636770.png)
![Ir[dFMeppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/355/743/1aef4eef-372d-4f1d-8904-08df7c4fd417/640/1aef4eef-372d-4f1d-8904-08df7c4fd417.png)



![[Ru(bpz)3][PF6]2 95%](/deepweb/assets/sigmaaldrich/product/structures/317/925/f0ef928e-bbea-4535-abe6-dda0bc28d32a/640/f0ef928e-bbea-4535-abe6-dda0bc28d32a.png)
![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
![Ir[p-F(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)