900811
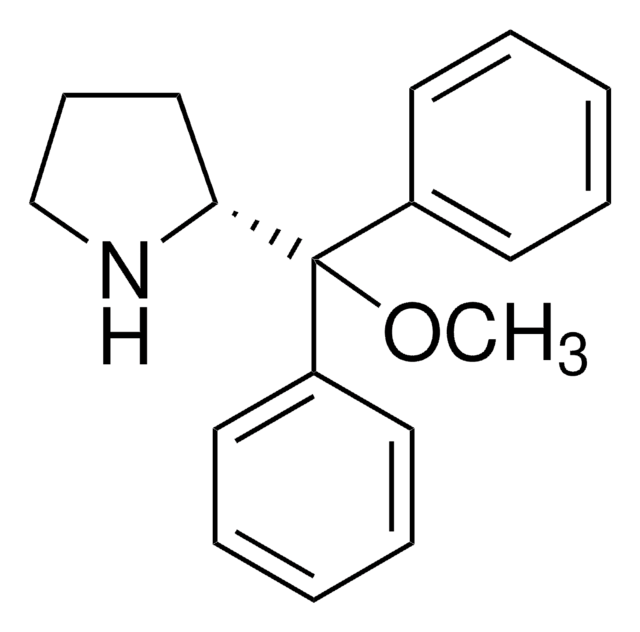
(S)-2-(2,3-Bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride
≥95%
Synonym(s):
(βS)-β-[[2,3-bis(dicyclohexylamino)-2-cyclopropen-1-ylidene]amino]-benzenepropanol hydrochloride (1:1), Dicyclohexyl cyclopropenimine, Lambert cyclopropenimine catalyst
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder or solid
reaction suitability
reagent type: catalyst
reaction type: Asymmetric synthesis
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
functional group
amine
hydroxyl
imine
phenyl
greener alternative category
, Aligned
General description
Application
Other Notes
Cyclopropenimine-Catalyzed Enantioselective Mannich Reactions of tert-Butyl Glycinates with N-Boc-Imines
Transition State Analysis of Enantioselective Bronsted Base Catalysis by Chiral Cyclopropenimines
Structure-activity relationship studies of cyclopropenimines as enantioselective Bronsted base catalysts
Asymmetric Bronsted Base-Catalyzed and -Directed [3+2] Cycloaddition of 2-Acyl Cycloheptatrienes with Azomethine Ylides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service