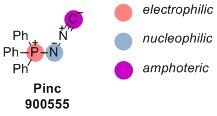
900555
(N-Isocyanoimino)triphenylphosphorane
95%
Synonym(s):
Pinc
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
application(s)
peptide synthesis
SMILES string
[P](=N[N+]#[C-])(c3ccccc3)(c2ccccc2)c1ccccc1
InChI
1S/C19H15N2P/c1-20-21-22(17-11-5-2-6-12-17,18-13-7-3-8-14-18)19-15-9-4-10-16-19/h2-16H
InChI key
NIDTXBFHPXMXTR-UHFFFAOYSA-N
Related Categories
General description
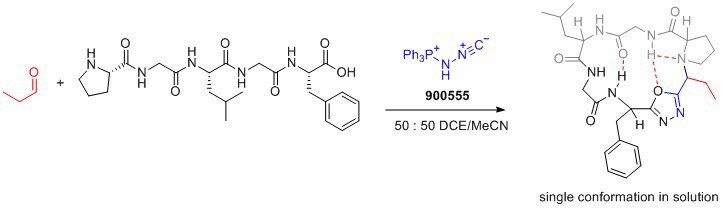
Application
Features and Benefits
- Bench-stable solid: Pinc is a stable reagent that can be easily handled and stored.
- Facilitates cyclization and incorporation of conformational control element: Pinc enables the formation of peptide macrocycles with a desired conformation, leading to improved drug properties.
- Amphoteric properties: Pinc′s amphoteric nature allows for the design of new multicomponent reactions, expanding its synthetic capabilities.
- Desired drug properties: The resulting peptide macrocycles exhibit enhanced membrane permeability, lipophilicity, and aqueous solubility, making them desirable for drug development.
- Versatile transformations: Pinc′s ability to form oxadiazoles opens up opportunities for the development of novel synthetic transformations.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Isocyanides are widely used reagents in organic synthesis, with applications ranging from materials science to drug discovery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)