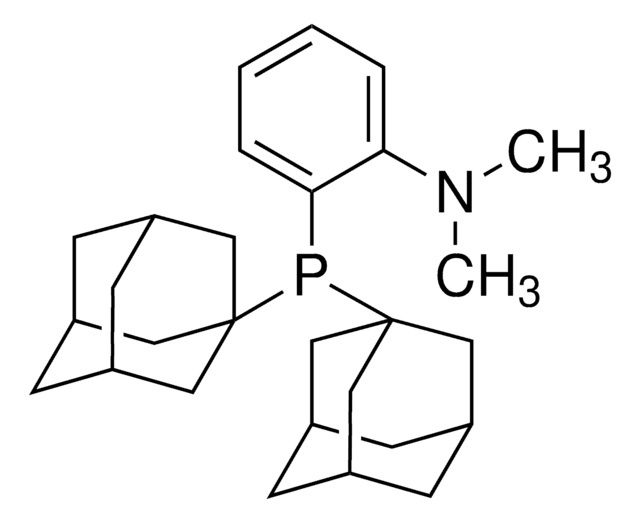
751618
MorDalphos
97%
Synonym(s):
Di(1-adamantyl)-2-morpholinophenylphosphine
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
reaction suitability
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
mp
219-224 °C
functional group
phosphine
storage temp.
2-8°C
SMILES string
C1CN(CCO1)c2ccccc2P([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C30H42NOP/c1-2-4-28(27(3-1)31-5-7-32-8-6-31)33(29-15-21-9-22(16-29)11-23(10-21)17-29)30-18-24-12-25(19-30)14-26(13-24)20-30/h1-4,21-26H,5-20H2/t21-,22+,23-,24-,25+,26-,29-,30-
InChI key
CCBRRSUORFMQCZ-BVIFAWIJSA-N
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
DalPhos is air-stable and contains the bulky di(1-adamantyl)phosphino [P(1-Ad)2] fragment. These ligands are useful in Pd-catalyzed C-N and C-C bond formation. Both Me-DalPhos and Mor-DalPhos allow for Pd-catalyzed ammonia, hydrazine and acetone cross-coupling with good functional group tolerance
Related Content
The main focus of the Stradiotto group has centered on the development and application of novel electronically rich, sterically encumbered P,N-based ancillary ligands for use in late metal catalysis, including Pd and Au chemistry.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
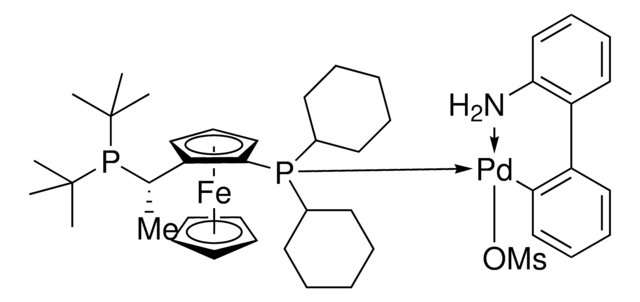
![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)