All Photos(1)
About This Item
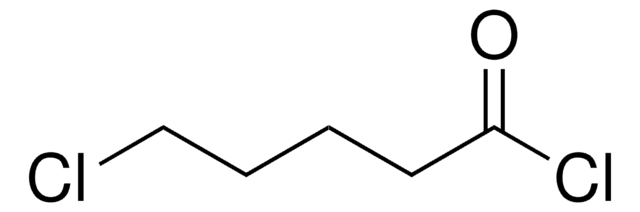
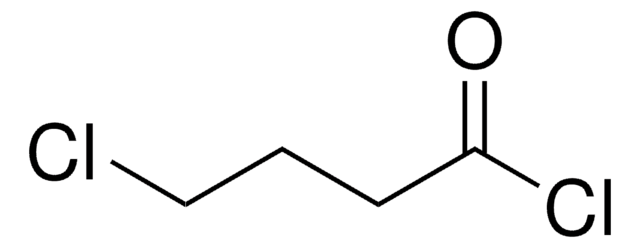
Linear Formula:
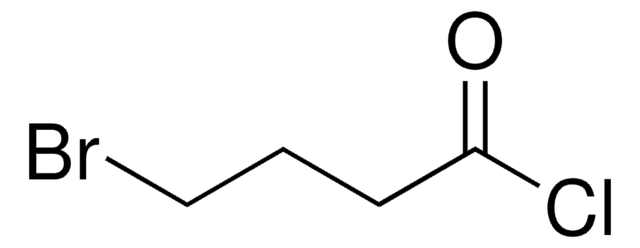
Cl(CH2)5COCl
CAS Number:
Molecular Weight:
169.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.4640 (lit.)
density
1.400 g/mL at 25 °C (lit.)
functional group
acyl chloride
chloro
SMILES string
ClCCCCCC(Cl)=O
InChI
1S/C6H10Cl2O/c7-5-3-1-2-4-6(8)9/h1-5H2
InChI key
WZILXAPNPKMOSA-UHFFFAOYSA-N
Application
6-Chlorohexanoyl chloride may be used in the following processes:
- Preparation of tert-butyl-N-(2-(6-chlorovaleryl)phenyl)carbamate, an intermediate for synthesizing the ketone analog of 2-amino-N-(4-(4-(1,2-benzisothiazol-3-yl)-1-piperazinyl)-butyl)benzamide hydrochloride.
- Preparation of (S)-(-)6-chlorohexanoic acid (2-methoxymethylpyrrolidin-1-yl)amide, a precursor for synthesizing 2-alkyl-substituted lactones.
- Deprotection and acylation of the pendant nitrogen during the preparation of a new cryptand with hydroxamate moiety that can bind with tribasic cations.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Analogues of the potential antipsychotic agent 1192U90: amide modifications.
Navas III F, et al.
Bioorganic & Medicinal Chemistry, 6(6), 811-823 (1998)
Enantioselective Synthesis of 2-Substituted 6-and 7-Membered Lactones via a-Alkylation of Lactone Hydrazones.
Enders D, et al.
Synthesis, 1995(08), 947-951 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service