All Photos(1)
About This Item
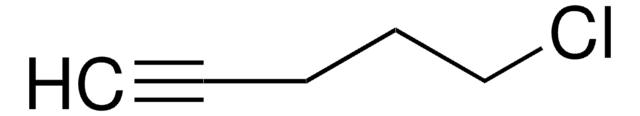
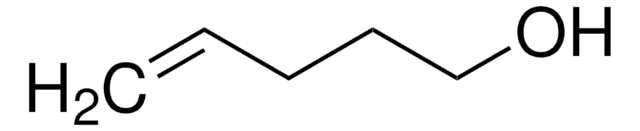
Linear Formula:
Cl(CH2)4CH=CH2
CAS Number:
Molecular Weight:
118.60
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.438 (lit.)
bp
135-136 °C (lit.)
density
0.896 g/mL at 25 °C (lit.)
functional group
alkyl halide
allyl
chloro
SMILES string
ClCCCCC=C
InChI
1S/C6H11Cl/c1-2-3-4-5-6-7/h2H,1,3-6H2
InChI key
BLMIXWDJHNJWDT-UHFFFAOYSA-N
Related Categories
General description
6-Chloro-1-hexene is an ω-chloro-1-alkene. It reacts with β-substituted imines in the presence of a rhodium catalyst to afford tri- and tetra-substituted imines. It undergoes cross-coupling reaction in the presence of copper(II) chloride and 1-phenylpropyne.
Application
6-Chloro-1-hexene may be used in the synthesis of 1-(5-hexen)-2,3-dimethyl-imidazolium chloride.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
84.0 °F - closed cup
Flash Point(C)
28.9 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Signs of Si29-H1 and Si29-F19 Coupling Constants
Danyluk SS.
Journal of the American Chemical Society, 86.20, 4504-4505 (1964)
Stereoselective alkylation of a, ?-unsaturated imines via CH bond activation
Colby, Denise A., Robert G. Bergman, and Jonathan A. Ellman
Journal of the American Chemical Society, 128.17, 5604-5605 (2006)
Copper nanoparticle-catalyzed cross-coupling of alkyl halides with Grignard reagents.
Kim JH and Chung YK.
Chemical Communications (Cambridge, England), 49(94), 11101-11103 (2013)
New ionic liquid-modified silica gels as recyclable materials for l-proline-or H?Pro?Pro?Asp?NH2-catalyzed aldol reaction
Aprile C, et al.
Green Chemistry, 9.12, 1328-1334 (2007)
Christopher L Rock et al.
Dalton transactions (Cambridge, England : 2003), 48(2), 461-467 (2018-11-30)
The phosphine-substituted α-diimine Ni precursor, (Ph2PPrDI)Ni, has been found to catalyze alkene hydrosilylation in the presence of Ph2SiH2 with turnover frequencies of up to 124 h-1 at 25 °C (990 h-1 at 60 °C). Moreover, the selective hydrosilylation of allylic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service