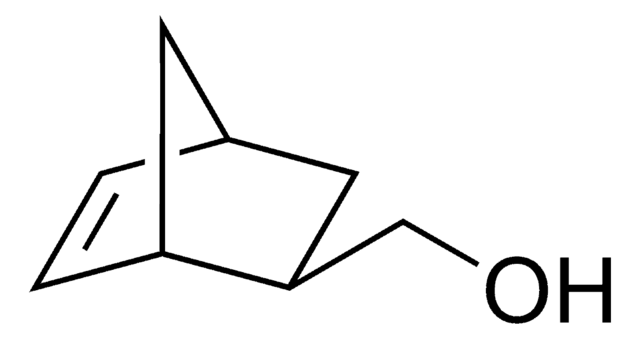
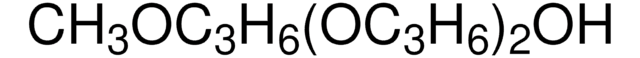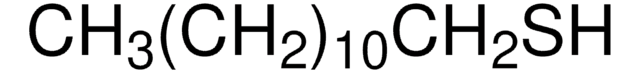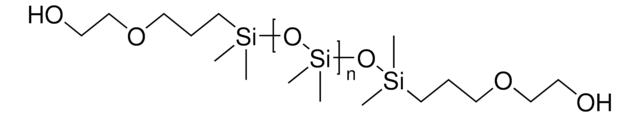525200
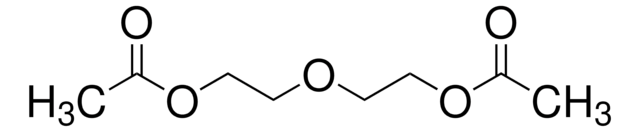
Ethylene glycol diacetate
99%
Synonym(s):
1,2-Diacetoxyethane, Ethylene diacetate, Glycol diacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
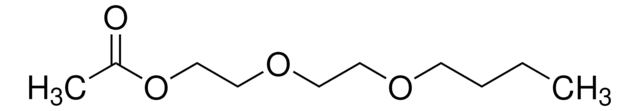
Linear Formula:
CH3COOCH2CH2OCOCH3
CAS Number:
Molecular Weight:
146.14
Beilstein:
1762308
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5.04 (vs air)
Quality Level
vapor pressure
0.2 mmHg ( 20 °C)
Assay
99%
autoignition temp.
899 °F
expl. lim.
1.6 %, 135 °F
8.4 %, 154 °F
refractive index
n20/D 1.415 (lit.)
bp
186-187 °C (lit.)
mp
−41 °C (lit.)
density
1.104 g/mL at 20 °C (lit.)
functional group
ester
SMILES string
CC(=O)OCCOC(C)=O
InChI
1S/C6H10O4/c1-5(7)9-3-4-10-6(2)8/h3-4H2,1-2H3
InChI key
JTXMVXSTHSMVQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethylene glycol diacetate (EGDA) is an acetic acid ester of ethylene glycol. It is widely used as a hardener for silicates. The predicted values of the torsion angles and bond angles are found to be in agreement with the crystal structure data on the benzoate derivatives of ethylene glycol. EGDA can be prepared from ethylene glycol and acetic acid, via esterification in the presence of supported ionic liquids (catalyst). It is a substitute of glyceryl triacetate for the industrial manufacture of tobacco.
Application
Ethylene glycol diacetate may be used as an acyl donor for the in situ generation of peracetic acid, during the chemoenzymatic synthesis of caprolactone. It may be employed as a precursor for the enzymatic synthesis of poly (ethylene glutarate).
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
181.4 °F - closed cup
Flash Point(C)
83 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Study on the esterification for ethylene glycol diacetate using supported ionic liquids as catalyst: Catalysts preparation, characterization, and reaction kinetics, process.
Yang J, et al.
Chemical Engineering Journal, 280, 147-157 (2015)
Xiaoman Zhao et al.
Ultrasonics sonochemistry, 31, 506-511 (2016-03-12)
The present work explores the best conditions for the enzymatic synthesis of poly (ethylene glutarate) for the first time. The start-up materials are the liquids; diethyl glutarate and ethylene glycol diacetate, without the need of addition of extra solvent. The
The toxic compounds and leaching characteristics of spent foundry sands.
Ji S, et al.
Water, Air, and Soil Pollution, 132(3-4), 347- 364 (2001)
Baeyer?Villiger Oxidation of Cyclohexanone in Aqueous Medium with In Situ Generation of Peracid Catalyzed by Perhydrolase CLEA.
Chavez G, et al.
Topics in Catalysis, 57(5), 349-355 (2014)
Conformational Studies on Oligomethylene Glycol Derivatives III. Ethylene Glycol Diacetate.
Sundararajan PR, et al.
Canadian Journal of Chemistry, 53(23), 3557-3562 (1975)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service