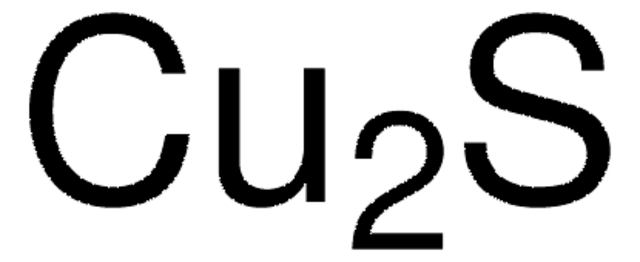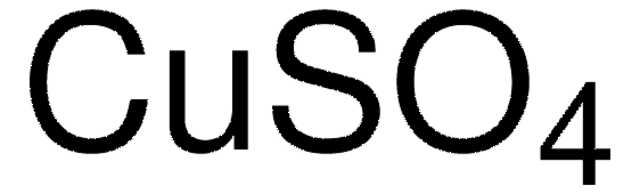510653
Copper(I) sulfide
anhydrous, powder, 99.99% trace metals basis
Synonym(s):
Chalcocite, Copper(1+) sulfide, Dicopper monosulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
Cu2S
CAS Number:
Molecular Weight:
159.16
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
anhydrous
Assay
99.99% trace metals basis
form
powder
technique(s)
NMR: suitable
impurities
≤150.0 ppm Trace Metal Analysis
density
5.6 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
[Cu]S[Cu]
InChI
1S/2Cu.S
InChI key
JESPAFKOCOFQIN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Cu63 NMR, X ray photoelectron spectroscopy,2 copper sulfide CuS was studied in detail. Copper sulfide is a monovalent and oxidation state was determined to be 2.2
Packaging
Packaged in ampules
accessory
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lidia Armelao et al.
Journal of nanoscience and nanotechnology, 6(2), 401-408 (2006-04-01)
A novel method for the preparation of CuS nanoparticles based on the fast nucleation of the sulphide has been developed. The particles have been synthesized by reaction of thioacetic acid with water and copper carboxylates (acetate, propionate) in the corresponding
Riccardo Narizzano et al.
The journal of physical chemistry. B, 109(33), 15798-15802 (2006-07-21)
A heterostructure formed by a conjugated polymer and semiconducting nanoparticles was produced. The conjugated polymer was synthesized by oxidative copolymerization of 3-thiopheneacetic acid and 3-hexylthiophene, thus obtaining an amphiphilic polythiophene that allows the formation of a stable polymer layer at
Yuming Guo et al.
Chemical communications (Cambridge, England), 46(20), 3493-3495 (2010-04-09)
Copper sulfide amorphous nanoparticles and nanocrystals were prepared successfully by a special process. These CuS nanoparticles could specifically and significantly induce the apoptosis and inhibit the proliferation of human cancer cells rather than normal cells. Moreover, the biological activities of
Xiangjie Bo et al.
Talanta, 81(1-2), 339-345 (2010-03-02)
A simple and facile synthetic method to incorporate copper sulfide (Cu(2)S) nanoparticles inside the mesopores of ordered mesoporous carbons (OMCs) is reported. The Cu(2)S/OMCs nanocomposite was characterized by transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and
Understanding ionic vacancy diffusion growth of cuprous sulfide nanowires.
Xiaohua Liu et al.
Angewandte Chemie (International ed. in English), 49(18), 3165-3168 (2010-03-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service