476099
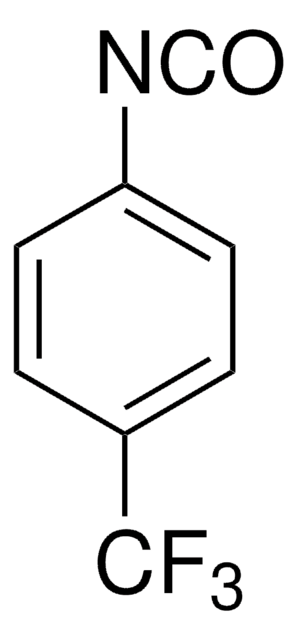
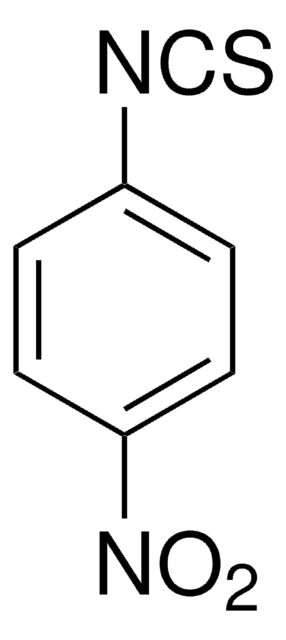
4-(Trifluoromethyl)phenyl isothiocyanate
97%
Synonym(s):
1-Isothiocyanato-4-(trifluoromethyl)benzene, 1-Isothiocyanato-4-trifluoromethylbenzene, p-Trifluoromethylphenyl isothiocyanate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CF3C6H4NCS
CAS Number:
Molecular Weight:
203.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
81 °C/11 mmHg (lit.)
mp
39-43 °C (lit.)
functional group
fluoro
isothiocyanate
SMILES string
FC(F)(F)c1ccc(cc1)N=C=S
InChI
1S/C8H4F3NS/c9-8(10,11)6-1-3-7(4-2-6)12-5-13/h1-4H
InChI key
DQEVDFQAYLIBRD-UHFFFAOYSA-N
Related Categories
General description
4-(Trifluoromethyl)phenyl isothiocyanate, also referred as p-trifluoromethylphenylisothiocyanate, is an isothiocyanate derivative.
Application
4-(Trifluoromethyl)phenyl isothiocyanate may be used in the synthesis of 6-[1-amino-3-(4-trifluoromethylphenyl)-thiourea]-2-ethylbenzo[de]isoquinoline-1,3-dione. It may also be used in the synthesis of photoinduced electron transfer (PET) sensors.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thorfinnur Gunnlaugsson et al.
The Journal of organic chemistry, 70(26), 10875-10878 (2005-12-17)
[structure: see text] The synthesis and UV-vis and NMR spectroscopic studies of thiourea-based colorimetric sensors for anions are presented. These sensors can recognize anions through hydrogen binding even in competitive pH-buffered aqueous solutions, giving rise to large color changes that
Emma B Veale et al.
Organic & biomolecular chemistry, 7(17), 3447-3454 (2009-08-14)
The thiourea based 4-amino-1,8-naphthalimide molecules 1-5 were designed as fluorescent anion sensors and their photophysical properties investigated upon recognition of biologically relevant anions such as acetate, dihydrogen phosphate and fluoride in DMSO. Synthesised in a single step from their respective
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service