437379
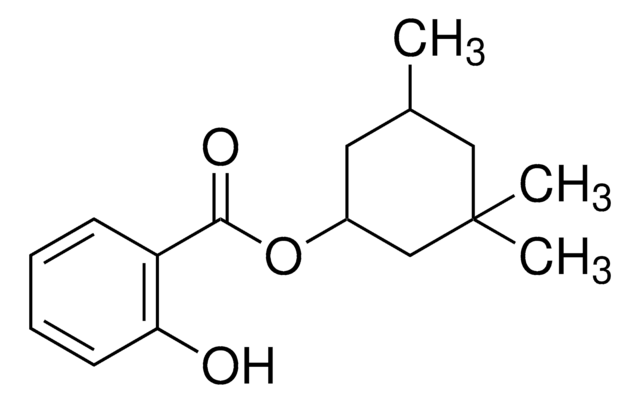
2-Ethylhexyl salicylate
99%
Synonym(s):
Octisalate, Octyl salicylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)C6H4CO2CH2CH(C2H5)(CH2)3CH3
CAS Number:
Molecular Weight:
250.33
Beilstein:
2730664
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
189-190 °C/21 mmHg (lit.)
density
1.014 g/mL at 25 °C (lit.)
SMILES string
CCCCC(CC)COC(=O)c1ccccc1O
InChI
1S/C15H22O3/c1-3-5-8-12(4-2)11-18-15(17)13-9-6-7-10-14(13)16/h6-7,9-10,12,16H,3-5,8,11H2,1-2H3
InChI key
FMRHJJZUHUTGKE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Ethylhexyl salicylate(EHS) is an organic ultraviolet(UV) absorber that can be used as a photostable ingredient in cosmetic formulations. It shows an absorption spectra in the range of 280-320 nm in the UV region.
Application
EHS is a salicylic ester which can be used as an UV filter in sunscreen based creams.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
323.6 °F - closed cup
Flash Point(C)
162 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Optical and electron paramagnetic resonance studies of the excited triplet states of UV-B absorbers: 2-ethylhexyl salicylate and homomenthyl salicylate.
Sugiyama K, et al.
Photochemical & Photobiological Sciences : Official Journal of the European Photochemistry Association and the European Society for Photobiology, 14(9), 1651-1659 (2015)
A pendant peptide endows a sunscreen with water-resistance.
Ellison AJ and Raines RT
Organic & Biomolecular Chemistry, 16(39), 7139-7142 (2018)
M McVean et al.
Molecular carcinogenesis, 24(3), 169-176 (1999-04-16)
Topical application of alpha-tocopherol (alphaTH), the most prominent naturally occurring form of vitamin E, inhibits ultraviolet (UV) B-induced photocarcinogenesis and DNA photodamage in C3H mice in vivo. In this study, we compared alphaTH with other vitamin E compounds and with
R Jiang et al.
Journal of pharmaceutical sciences, 86(7), 791-796 (1997-07-01)
This study provides an investigation of the availability of octyl salicylate (OS), a common sunscreen agent, from liquid paraffin and the effect of OS on skin permeability. A model membrane system to isolate the vehicle effect from membrane permeability has
M McVean et al.
Carcinogenesis, 18(8), 1617-1622 (1997-08-01)
Ultraviolet B (UVB, 290-320 nm) exposure results in a variety of cellular insults including induction of cyclobutane pyrimidine dimers in DNA. Accumulation of these lesions can lead to mutations in critical genes and contribute to the development of nonmelanoma skin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Hexyl 2-[4-(diethylamino)-2-hydroxybenzoyl]benzoate analytical standard](/deepweb/assets/sigmaaldrich/product/structures/171/239/18149257-0ac2-4ee5-8e7c-b0086ca9ee81/640/18149257-0ac2-4ee5-8e7c-b0086ca9ee81.png)